Abstract
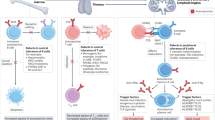
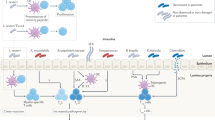
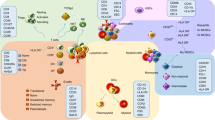
Autoimmune diseases are a diverse group of conditions characterized by aberrant B cell and T cell reactivity to normal constituents of the host. These diseases occur widely and affect individuals of all ages, especially women. Among these diseases, the most prominent immunological manifestation is the production of autoantibodies, which provide valuable biomarkers for diagnosis, classification and disease activity. Although T cells have a key role in pathogenesis, they are technically more difficult to assay. In general, autoimmune disease results from an interplay between a genetic predisposition and environmental factors. Genetic predisposition to autoimmunity is complex and can involve multiple genes that regulate the function of immune cell populations. Less frequently, autoimmunity can result from single-gene mutations that affect key regulatory pathways. Infection seems to be a common trigger for autoimmune disease, although the microbiota can also influence pathogenesis. As shown in seminal studies, patients may express autoantibodies many years before the appearance of clinical or laboratory signs of disease — a period called pre-clinical autoimmunity. Monitoring autoantibody expression in at-risk populations may therefore enable early detection and the initiation of therapy to prevent or attenuate tissue damage. Autoimmunity may not be static, however, and remission can be achieved by some patients treated with current agents.
Key points
-
Autoimmune diseases lead to diverse patterns of inflammation and organ dysfunction.
-
Autoantibodies are valuable markers for diagnosis, classification and of disease activity.
-
Although T cells play a key part in disease, their assessment is challenging.
-
Autoimmune disease reflects the interplay of genetic and environmental factors.
-
Pre-clinical autoimmune disease provides a window of time for early or preventive treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Slight-Webb, S., Bourn, R. L., Holers, V. M. & James, J. A. Shared and unique immune alterations in pre-clinical autoimmunity. Curr. Opin. Immunol. 61, 60–68 (2019).
Sethi, S., De Vriese, A. S. & Fervenza, F. C. Acute glomerulonephritis. Lancet 399, 1646–1663 (2022).
Beck, L. H. Jr. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Sethi, S. New ‘antigens’ in membranous nephropathy. J. Am. Soc. Nephrol. 32, 268–278 (2021).
Watts, A. J. B. et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J. Am. Soc. Nephrol. 33, 238–252 (2022).
Rose, N. R. & Bona, C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol. Today 14, 426–430 (1993).
Mecoli, C. A. & Casciola-Rosen, L. An update on autoantibodies in scleroderma. Curr. Opin. Rheumatol. 30, 548–553 (2018).
McHugh, N. J. & Tansley, S. L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 14, 290–302 (2018).
Lazaridis, K. & Tzartos, S. J. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front. Immunol. 11, 212 (2020).
Moore, E. et al. Promise and complexity of lupus mouse models. Nat. Immunol. 22, 683–686 (2021).
de Moel, E. C. et al. In rheumatoid arthritis, changes in autoantibody levels reflect intensity of immunosuppression, not subsequent treatment response. Arth. Res. Ther. 21, 28 (2019).
van de Logt, A. E. et al. Immunological remission in PLA2R-antibody-associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 93, 1016–1017 (2018).
Wu, W. et al. The prognostic value of phospholipase A2 receptor autoantibodies on spontaneous remission for patients with idiopathic membranous nephropathy: a meta-analysis. Medicine 97, e11018 (2018).
Goebel, A. et al. The autoimmune aetiology of unexplained chronic pain. Autoimmun. Rev. 21, 103015 (2022).
Goebel, A. et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Investig. https://doi.org/10.1172/jci144201 (2021).
Pollak, T. A. et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiat. 7, 93–108 (2020).
Larman, H. B. et al. Autoantigen discovery with a synthetic human peptidome. Nat. Biotechnol. 29, 535–541 (2011).
Wang, E. Y. et al. High-throughput identification of autoantibodies that target the human exoproteome. Cell Rep. Meth. https://doi.org/10.1016/j.crmeth.2022.100172 (2022).
Knight, J. S. et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. https://doi.org/10.1172/jci154886 (2021).
Shome, M. et al. Serum autoantibodyome reveals that healthy individuals share common autoantibodies. Cell Rep. 39, 110873 (2022).
Caza, T. N. et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 100, 171–181 (2021).
Ronco, P. & Debiec, H. Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J. Am. Soc. Nephrol. 16, 1205–1213 (2005).
Graus, F., Saiz, A. & Dalmau, J. GAD antibodies in neurological disorders — insights and challenges. Nat. Rev. Neurol. 16, 353–365 (2020).
Buzzetti, R. et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes 69, 2037–2047 (2020).
Rüster, M., Kiehntopf, M., Gröne, H. J. & Wolf, G. A Friday afternoon case of apparent anti-glomerular basement nephritis. Nephrol. Dial. Transplant. 21, 2328–2330 (2006).
Fritzler, M. J., Choi, M. Y., Satoh, M. & Mahler, M. Autoantibody discovery, assay development and adoption: death valley, the sea of survival and beyond. Front. Immunol. 12, 679613 (2021).
Pisetsky, D. S. & Lipsky, P. E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 16, 565–579 (2020).
Rosen, A. & Casciola-Rosen, L. Autoantigens as partners in initiation and propagation of autoimmune rheumatic diseases. Annu. Rev. Immunol. 34, 395–420 (2016).
Scherer, H. U., Huizinga, T. W. J., Krönke, G., Schett, G. & Toes, R. E. M. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat. Rev. Rheumatol. 14, 157–169 (2018).
Mitchell, A. M. et al. T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2019129118 (2021).
Poppelaars, F. & Thurman, J. M. Complement-mediated kidney diseases. Mol. Immunol. 128, 175–187 (2020).
Kant, S., Kronbichler, A., Sharma, P. & Geetha, D. Advances in understanding of pathogenesis and treatment of immune-mediated kidney disease: a review. Am. J. Kidney Dis. 79, 582–600 (2022).
Zykova, S. N., Tveita, A. A. & Rekvig, O. P. Renal Dnase1 enzyme activity and protein expression is selectively shut down in murine and human membranoproliferative lupus nephritis. PLoS One https://doi.org/10.1371/journal.pone.0012096 (2010).
Jayne, D. R. W., Merkel, P. A., Schall, T. J. & Bekker, P. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
Alba, M. A., Jennette, J. C. & Falk, R. J. Pathogenesis of ANCA-associated pulmonary vasculitis. Semin. Respir. Crit. Care Med. 39, 413–424 (2018).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7, e32366 (2012).
Kasperkiewicz, M. et al. Pemphigus. Nat. Rev. Dis. Prim. 3, 17026 (2017).
Wang, E. Y. et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021).
Puel, A., Bastard, P., Bustamante, J. & Casanova, J. L. Human autoantibodies underlying infectious diseases. J. Exp. Med. https://doi.org/10.1084/jem.20211387 (2022).
Beurskens, F. J., van Schaarenburg, R. A. & Trouw, L. A. C1q, antibodies and anti-C1q autoantibodies. Mol. Immunol. 68, 6–13 (2015).
Bomback, A. S. et al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 93, 977–985 (2018).
Yoshitomi, H. & Ueno, H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell. Mol. Immunol. 18, 523–527 (2021).
den Braanker, D. J. W. et al. Novel in vitro assays to detect circulating permeability factor(s) in idiopathic focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 36, 247–256 (2021).
Sims, E. K., Mirmira, R. G. & Evans-Molina, C. The role of beta-cell dysfunction in early type 1 diabetes. Curr. Opin. Endocrinol. Diab. Obes. 27, 215–224 (2020).
Hogquist, K. A., Baldwin, T. A. & Jameson, S. C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782 (2005).
Bluestone, J. A. & Anderson, M. Tolerance in the age of immunotherapy. N. Engl. J. Med. 383, 1156–1166 (2020).
Tsubata, T. B-cell tolerance and autoimmunity. F1000 Res. 6, 391 (2017).
Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 17, 281–294 (2017).
Takaba, H. & Takayanagi, H. The mechanisms of T cell selection in the thymus. Trends Immunol. 38, 805–816 (2017).
Anderson, M. S. & Su, M. A. AIRE expands: new roles in immune tolerance and beyond. Nat. Rev. Immunol. 16, 247–258 (2016).
Takaba, H. et al. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell 163, 975–987 (2015).
Meffre, E. & O’Connor, K. C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 292, 90–101 (2019).
Rawlings, D. J., Metzler, G., Wray-Dutra, M. & Jackson, S. W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 17, 421–436 (2017).
Mintz, M. A. & Cyster, J. G. T follicular helper cells in germinal center B cell selection and lymphomagenesis. Immunol. Rev. 296, 48–61 (2020).
Jamaly, S., Rakaee, M., Abdi, R., Tsokos, G. C. & Fenton, K. A. Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev. 20, 102980 (2021).
ElTanbouly, M. A. & Noelle, R. J. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat. Rev. Immunol. 21, 257–267 (2021).
Wing, J. B., Tanaka, A. & Sakaguchi, S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50, 302–316 (2019).
Shevach, E. M. Foxp3+ T regulatory cells: still many unanswered questions — A perspective after 20 years of study. Front. Immunol. 9, 1048 (2018).
Dasgupta, S., Dasgupta, S. & Bandyopadhyay, M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell. Immunol. 352, 104076 (2020).
Criswell, L. A. et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am. J. Hum. Genet. 76, 561–571 (2005).
Parkes, M., Cortes, A., van Heel, D. A. & Brown, M. A. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat. Rev. Genet. 14, 661–673 (2013).
Iwamoto, T. & Niewold, T. B. Genetics of human lupus nephritis. Clin. Immunol. 185, 32–39 (2017).
Robertson, C. C. et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat. Genet. 53, 962–971 (2021).
Dendrou, C. A., Petersen, J., Rossjohn, J. & Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339 (2018).
Goulielmos, G. N. et al. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene 668, 59–72 (2018).
Estrada, K. et al. A whole-genome sequence study identifies genetic risk factors for neuromyelitis optica. Nat. Commun. 9, 1929 (2018).
Lenz, T. L. et al. Widespread non-additive and interaction effects within HLA loci modulate the risk of autoimmune diseases. Nat. Genet. 47, 1085–1090 (2015).
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
Cano-Gamez, E. & Trynka, G. From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 11, 424 (2020).
Benaglio, P. et al. Type 1 diabetes risk genes mediate pancreatic beta cell survival in response to proinflammatory cytokines. Cell Genom. 2, 100214 (2022).
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47, 1457–1464 (2015).
Coit, P. et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naïve CD4+ T cells from lupus patients. J. Autoimmun. 43, 78–84 (2013).
Coit, P. et al. Epigenetic reprogramming in naive CD4+ T cells favoring T cell activation and non-Th1 effector T cell immune response as an early event in lupus flares. Arth. Rheumatol. 68, 2200–2209 (2016).
Stanford, S. M. & Bottini, N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat. Rev. Rheumatol. 10, 602–611 (2014).
Mustelin, T., Bottini, N. & Stanford, S. M. The contribution of PTPN22 to rheumatic disease. Arth. Rheumatol. 71, 486–495 (2019).
Tizaoui, K. et al. The role of PTPN22 in the pathogenesis of autoimmune diseases: a comprehensive review. Semin. Arth. Rheum. 51, 513–522 (2021).
Xie, J. et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat. Commun. 11, 1600 (2020).
Stanescu, H. C. et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364, 616–626 (2011).
Catalina, M. D. et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight https://doi.org/10.1172/jci.insight.140380 (2020).
Langefeld, C. D. et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat. Commun. 8, 16021 (2017).
Menard, L. C. et al. B cells from African American lupus patients exhibit an activated phenotype. JCI Insight 1, e87310 (2016).
Owen, K. A. et al. Analysis of trans-ancestral SLE risk loci identifies unique biologic networks and drug targets in African and European ancestries. Am. J. Hum. Genet. 107, 864–881 (2020).
Tesar, V. & Hruskova, Z. Lupus nephritis: a different disease in European patients. Kidney Dis. 1, 110–118 (2015).
Isenberg, D. et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology 49, 128–140 (2010).
Yusuf, A. A., Govender, M. A., Brandenburg, J. T. & Winkler, C. A. Kidney disease and APOL1. Hum. Mol. Genet. 30, R129–r137 (2021).
Omarjee, O. et al. Monogenic lupus: dissecting heterogeneity. Autoimmun. Rev. 18, 102361 (2019).
Zipfel, P. F., Wiech, T., Stea, E. D. & Skerka, C. CFHR gene variations provide insights in the pathogenesis of the kidney diseases atypical hemolytic uremic syndrome and C3 glomerulopathy. J. Am. Soc. Nephrol. 31, 241–256 (2020).
Crow, Y. J. & Stetson, D. B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. 22, 471–483 (2022).
Husebye, E. S., Anderson, M. S. & Kämpe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 1132–1141 (2018).
Ferre, E. M. et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy. JCI Insight https://doi.org/10.1172/jci.insight.88782 (2016).
Barzaghi, F. & Passerini, L. IPEX syndrome: improved knowledge of immune pathogenesis empowers diagnosis. Front. Pediatr. 9, 612760 (2021).
Cepika, A. M. et al. Tregopathies: monogenic diseases resulting in regulatory T-cell deficiency. J. Allergy Clin. Immunol. 142, 1679–1695 (2018).
Lundtoft, C. et al. Complement C4 copy number variation is linked to SSA/Ro and SSB/La autoantibodies in systemic inflammatory autoimmune diseases. Arth. Rheumatol. 74, 1440–1450 (2022).
Hoshino, A. et al. Identification of autoantibodies using human proteome microarrays in patients with IPEX syndrome. Clin. Immunol. 203, 9–13 (2019).
Lampasona, V. et al. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One 8, e78664 (2013).
Souyris, M., Mejía, J. E., Chaumeil, J. & Guéry, J. C. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 41, 153–164 (2019).
Nusbaum, J. S. et al. Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin. Proc. 95, 384–394 (2020).
Webb, K. et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front. Immunol. 9, 3167 (2018).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. Usa. 111, 869–874 (2014).
Fischinger, S., Boudreau, C. M., Butler, A. L., Streeck, H. & Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 41, 239–249 (2019).
Scofield, R. H. et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arth. Rheum. 58, 2511–2517 (2008).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
Bergstra, S. A. et al. Sex-associated treatment differences and their outcomes in rheumatoid arthritis: results from the METEOR register. J. Rheumatol. 45, 1361–1366 (2018).
Warram, J. H., Krolewski, A. S., Gottlieb, M. S. & Kahn, C. R. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N. Engl. J. Med. 311, 149–152 (1984).
Bonifacio, E., Warncke, K., Winkler, C., Wallner, M. & Ziegler, A. G. Cesarean section and interferon-induced helicase gene polymorphisms combine to increase childhood type 1 diabetes risk. Diabetes 60, 3300–3306 (2011).
Dinse, G. E. et al. Increasing prevalence of antinuclear antibodies in the United States. Arth. Rheumatol. 72, 1026–1035 (2020).
Hermann, R. et al. Temporal changes in the frequencies of HLA genotypes in patients with type 1 diabetes — indication of an increased environmental pressure? Diabetologia 46, 420–425 (2003).
Gillespie, K. M. et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 364, 1699–1700 (2004).
Miller, A. L., Bessho, S., Grando, K. & Tükel, Ç. Microbiome or infections: amyloid-containing biofilms as a trigger for complex human diseases. Front. Immunol. 12, 638867 (2021).
Ruff, W. E., Greiling, T. M. & Kriegel, M. A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 18, 521–538 (2020).
Ogunrinde, E. et al. A link between plasma microbial translocation, microbiome, and autoantibody development in first-degree relatives of systemic lupus erythematosus patients. Arth. Rheumatol. 71, 1858–1868 (2019).
Jog, N. R. & James, J. A. Epstein Barr virus and autoimmune responses in systemic lupus erythematosus. Front. Immunol. 11, 623944 (2020).
Manasson, J., Blank, R. B. & Scher, J. U. The microbiome in rheumatology: where are we and where should we go? Ann. Rheum. Dis. 79, 727–733 (2020).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).
Artacho, A. et al. The pretreatment gut microbiome is associated with lack of response to methotrexate in new-onset rheumatoid arthritis. Arth. Rheumatol. 73, 931–942 (2021).
Azzouz, D. et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann. Rheum. Dis. 78, 947–956 (2019).
Pianta, A. et al. Identification of novel, immunogenic HLA-DR-presented Prevotella copri peptides in patients with rheumatoid arthritis. Arth. Rheumatol. 73, 2200–2205 (2021).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Yang, Y. et al. Within-host evolution of a gut pathobiont facilitates liver translocation. Nature 607, 563–570 (2022).
Mariño, E. et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017).
Zegarra-Ruiz, D. F. et al. A diet-sensitive commensal lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 25, 113–127.e116 (2019).
McPherson, A. C., Pandey, S. P., Bender, M. J. & Meisel, M. Systemic immunoregulatory consequences of gut commensal translocation. Trends Immunol. 42, 137–150 (2021).
Milligan, G., Shimpukade, B., Ulven, T. & Hudson, B. D. Complex pharmacology of free fatty acid receptors. Chem. Rev. 117, 67–110 (2017).
Brown, J., Robusto, B. & Morel, L. Intestinal dysbiosis and tryptophan metabolism in autoimmunity. Front. Immunol. 11, 1741 (2020).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40, 833–842 (2014).
Meier, H. C. S. et al. Hygiene hypothesis indicators and prevalence of antinuclear antibodies in US adolescents. Front. Immunol. 13, 789379 (2022).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arth. Rheum. 54, 38–46 (2006).
Tiniakou, E. & Christopher-Stine, L. Immune-mediated necrotizing myopathy associated with statins: history and recent developments. Curr. Opin. Rheumatol. 29, 604–611 (2017).
Mérida, E. & Praga, M. NSAIDs and nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 14, 1280–1282 (2019).
Burkett, J. B., Doran, A. C. & Gannon, M. Harnessing prostaglandin E(2) signaling to ameliorate autoimmunity. Trends Immunol. 44, 162–171 (2023).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 16, 535–548 (2019).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes. Curr. Opin. Neurol. 25, 795–801 (2012).
Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 23, 487–500 (2022).
Weinmann, S. C. & Pisetsky, D. S. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 58, vii59–vii67 (2019).
Olsen, N. J., Okuda, D. T., Holers, V. M. & Karp, D. R. Editorial: Understanding the concept of pre-clinical autoimmunity. Front. Immunol. 13, 983310 (2022).
Choi, M. Y. & Costenbader, K. H. Understanding the concept of pre-clinical autoimmunity: prediction and prevention of systemic lupus erythematosus: identifying risk factors and developing strategies against disease development. Front. Immunol. 13, 890522 (2022).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Sosenko, J. M. et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 36, 2615–2620 (2013).
Jacobsen, L. M. et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia 63, 588–596 (2020).
Haville, S. & Deane, K. D. Pre-RA: can early diagnosis lead to prevention? Best. Pract. Res. Clin. Rheumatol. 36, 101737 (2022).
Munroe, M. E. et al. Discerning risk of disease transition in relatives of systemic lupus erythematosus patients utilizing soluble mediators and clinical features. Arth. Rheumatol. 69, 630–642 (2017).
Munroe, M. E. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 75, 2014–2021 (2016).
Slight-Webb, S. et al. Autoantibody-positive healthy individuals with lower lupus risk display a unique immune endotype. J. Allergy Clin. Immunol. 146, 1419–1433 (2020).
So, M., O’Rourke, C., Bahnson, H. T., Greenbaum, C. J. & Speake, C. Autoantibody reversion: changing risk categories in multiple-autoantibody-positive individuals. Diab. Care 43, 913–917 (2020).
Powers, A. C. Type 1 diabetes mellitus: much progress, many opportunities. J. Clin. Investig. https://doi.org/10.1172/jci142242 (2021).
Dayan, C. M., Korah, M., Tatovic, D., Bundy, B. N. & Herold, K. C. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet 394, 1286–1296 (2019).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019).
Perdigoto, A. L. et al. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 62, 655–664 (2019).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Hirsch, J. S. FDA approves teplizumab: a milestone in type 1 diabetes. Lancet Diab. Endocrinol. 11, 18 (2023).
Deane, K. D. & Holers, V. M. Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift. Arth. Rheumatol. 73, 181–193 (2021).
Krijbolder, D. I. et al. Intervention with methotrexate in patients with arthralgia at risk of rheumatoid arthritis to reduce the development of persistent arthritis and its disease burden (TREAT EARLIER): a randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet 400, 283–294 (2022).
Arroyo, H. A. & Torres, A. R. Spontaneous remission in juvenile myasthenia gravis: a cohort of 13 cases and review of the literature. Neuromuscul. Disord. 32, 213–219 (2022).
Watanabe, S. et al. Spontaneous remission of thrombospondin type-1 domain-containing-associated membranous nephropathy. Intern. Med. 60, 3125–3128 (2021).
Dörner, T. & Lipsky, P. E. B cells: depletion or functional modulation in rheumatic diseases. Curr. Opin. Rheumatol. 26, 228–236 (2014).
Phalke, S. & Marrack, P. Age (autoimmunity) associated B cells (ABCs) and their relatives. Curr. Opin. Immunol. 55, 75–80 (2018).
Mouat, I. C., Goldberg, E. & Horwitz, M. S. Age-associated B cells in autoimmune diseases. Cell. Mol. Life Sci. 79, 402 (2022).
Vidal-Pedrola, G. et al. Characterization of age-associated B cells in early drug-naïve rheumatoid arthritis patients. Immunology https://doi.org/10.1111/imm.13598 (2022).
Wang, S. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018).
Smith, M. J., Cambier, J. C. & Gottlieb, P. A. Endotypes in T1D: B lymphocytes and early onset. Curr. Opin. Endocrinol. Diab. Obes. 27, 225–230 (2020).
Tipton, C. M. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Amanna, I. J. & Slifka, M. K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 236, 125–138 (2010).
Hale, M., Rawlings, D. J. & Jackson, S. W. The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr. Opin. Immunol. 55, 81–88 (2018).
Chang, H. D. et al. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol. 61, 86–91 (2019).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Ostendorf, L. et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N. Engl. J. Med. 383, 1149–1155 (2020).
Morris, A. D., Rowbottom, A. W., Martin, F. L., Woywodt, A. & Dhaygude, A. P. Biomarkers in ANCA-associated vasculitis: potential pitfalls and future prospects. Kidney360 2, 586–597 (2021).
Schur, P. H. & Sandson, J. Immunologic factors and clinical activity in systemic lupus erythematosus. N. Engl. J. Med. 278, 533–538 (1968).
McCarty, G. A., Rice, J. R., Bembe, M. L. & Pisetsky, D. S. Independent expression of autoantibodies in systemic lupus erythematosus. J. Rheumatol. 9, 691–695 (1982).
Schett, G. et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann. Rheum. Dis. 75, 1428–1437 (2016).
Wallace, D. J. et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arth. Rheum. 61, 1168–1178 (2009).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arth. Rheum. 63, 3918–3930 (2011).
Pisetsky, D. S., Rovin, B. H. & Lipsky, P. E. New perspectives in rheumatology: biomarkers as entry criteria for clinical trials of new therapies for systemic lupus erythematosus: the example of antinuclear antibodies and anti-DNA. Arth. Rheumatol. 69, 487–493 (2017).
Ludwig, R. J. et al. Mechanisms of autoantibody-induced pathology. Front. Immunol. 8, 603 (2017).
Barrat, F. J., Crow, M. K. & Ivashkiv, L. B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Philip Cohen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat Rev Nephrol 19, 509–524 (2023). https://doi.org/10.1038/s41581-023-00720-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00720-1
This article is cited by
-
Anti-inflammatory effects of cyclodextrin nanoparticles enable macrophage repolarization and reduce inflammation
Discover Nano (2024)
-
piRNA associates with immune diseases
Cell Communication and Signaling (2024)
-
Autoimmunity’s enigmatic role: exploring the connection with myalgic encephalomyelitis/chronic fatigue syndrome
BMC Immunology (2024)
-
Mesenchymal stromal cells restrain the Th17 cell response via L-amino-acid oxidase within lymph nodes
Cell Death & Disease (2024)
-
Long-term risk of autoimmune diseases after mRNA-based SARS-CoV2 vaccination in a Korean, nationwide, population-based cohort study
Nature Communications (2024)