Abstract
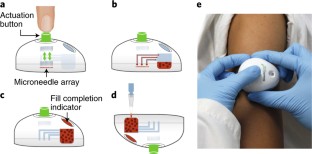
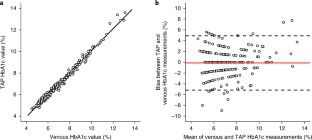
The advancement of point-of-care diagnostics and the decentralization of healthcare have created a need for the simple, safe, standardized and painless collection of blood specimens. Here, we describe the design and implementation of a capillary blood-collection device that is more convenient and less painful than a fingerstick and venepuncture, and collects 100 µl of blood. The technology integrates into a compact, self-contained device an array of solid microneedles, a high-velocity insertion mechanism, stored vacuum, and a microfluidic system containing lithium heparin anticoagulant. The use of the device requires minimal training, as blood collection is initiated by the single push of a button. In a clinical study involving 144 participants, haemoglobin A1c measurements from device-collected samples and from venous blood samples were equivalent, and the pain associated with the device was significantly less than that associated with venepuncture. The device, which has received premarket clearance by the US Food and Drug Administration, should help improve access to healthcare, and support healthcare decentralization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Windich-Biermeier, A., Sjoberg, I., Dale, J. C., Eshelman, D. & Guzzetta, C. E. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J. Pediatr. Oncol. Nurs. 24, 8–19 (2007).
Migdal, M., Chudzynska-Pomianowska, E., Vause, E., Henry, E. & Lazar, J. Rapid, needle-free delivery of lidocaine for reducing the pain of venipuncture among pediatric subjects. Pediatrics 115, e393–e398 (2005).
Hamilton, J. G. Needle phobia: a neglected diagnosis. J. Fam. Pract. 41, 169–176 (1995).
Deacon, B. & Abramowitz, J. Fear of needles and vasovagal reactions among phlebotomy patients. J. Anxiety Disord. 20, 946–960 (2006).
Chan, C. P. Y. et al. Evidence-based point-of-care diagnostics: current status and emerging technologies. Annu. Rev. Anal. Chem. 6, 191–211 (2013).
Yang, X. et al. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin. Chem. 59, 1506–1513 (2013).
Sølvik, U. Ø., Røraas, T., Christensen, N. G. & Sandberg, S. Diagnosing diabetes mellitus: performance of hemoglobin A1c point-of-care instruments in general practice offices. Clin. Chem. 59, 1790–1801 (2013).
Pollock, N. R. et al. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 4, 152ra129 (2012).
Kuriger, R. J. & Romanowicz, B. F. Analysis of blood extraction from a lancet puncture. in Tech. Proc. 2004 NSTI Nanotechnol. Conf. Trade Show 1, 275–279 (Nano Science and Technology Institute, 2004).
Wei, C.-H. et al. Clinical evaluation and alternative site blood glucose testing of the EasyPlus mini R2N blood glucose monitoring system. Clin. Chim. Acta 403, 167–172 (2009).
Cunningham, D. D. et al. Blood extraction from lancet wounds using vacuum combined with skin stretching. J. Appl. Physiol. 92, 1089–1096 (2002).
Heinemann, L. & Boecker, D. Lancing: quo vadis? J. Diabetes Sci. Technol. 5, 966–981 (2011).
Prausnitz, M. R. & Langer, R. Transdermal drug delivery. Nat. Biotechnol. 26, 1261–1268 (2008).
Donnelly, R. F., Singh, T. R. R. & Woolfson, A. D. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 17, 187–207 (2010).
Burton, S. A. et al. Rapid intradermal delivery of liquid formulations using a hollow microstructured array. Pharm. Res. 28, 31–40 (2011).
Patel, S. R., Lin, A. S. P., Edelhauser, H. F. & Prausnitz, M. R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 28, 166–176 (2011).
Coulman, S. A. et al. In Vivo, In Situ imaging of Microneedle Insertion into the Skin of Human Volunteers Using Optical Coherence Tomography. Pharm. Res. 28, 66–81 (2011).
Wang, P. M., Cornwell, M. G. & Prausnitz, M. R. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol. Ther. 7, 131–141 (2005).
Ventrelli, L., Marsilio Strambini, L. & Barillaro, G. Microneedles for transdermal biosensing: current picture and future direction. Adv. Healthc. Mater. 4, 2606–2640 (2015).
Li, C. G., Lee, C. Y., Lee, K. & Jung, H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Micro. 15, 17–25 (2013).
Ganesan, A. V. et al. Analysis of MEMS-based microneedles for blood monitoring. BioNanoScience 4, 128–135 (2014).
Li, C. G. et al. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip 15, 3286–3292 (2015).
Shergold, O. A. & Fleck, N. A. Mechanisms of deep penetration of soft solids, with application to the injection and wounding of skin. Proc. R. Soc. Lond. Math. Phys. Eng. Sci. 460, 3037–3058 (2004).
Davis, S. P., Landis, B. J., Adams, Z. H., Allen, M. G. & Prausnitz, M. R. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J. Biomech. 37, 1155–1163 (2004).
Crichton, M. L. et al. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials 31, 4562–4572 (2010).
Verbaan, F. J. et al. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J. Control. Release 128, 80–88 (2008).
Rousche, P. J. & Normann, R. A. A method of pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 20, 413–422 (1992).
The Physics of Diaphragm Springs (Häussermann, accessed 7 November 2017); http://www.haussermann.com/pub/pdf/en/tellerfeder.pdf
Braverman, I. M. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 5, 3–9 (2000).
Gill, H. S., Denson, D. D., Burris, B. A. & Prausnitz, M. R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 24, 585–594 (2008).
Davis, M. J., Demis, D. J. & Lawler, J. C. The microcirculation of the skin. J. Invest. Dermatol. 34, 31–35 (1960).
Landis, E. M. The capillaries of the skin. J. Invest. Dermatol. 1, 295–311 (1938).
Humbert, P., Fanian, F., Maibach, H. I. & Agache, P. Agache’s measuring the skin: non-invasive, investigations, physiology, normal constants (Springer, Berlin, Heidelberg, 2016).
Cunningham, D. D. et al. Vacuum-assisted lancing of the forearm: an effective and less painful approach to blood glucose monitoring. Diabetes Technol. Ther. 2, 541–548 (2000).
Simonen, P., O’Brien, M., Hamilton, C., Ashcroft, J. & Denham, J. Normal variation in cutaneous blood content and red blood cell velocity in humans. Physiol. Meas. 18, 155–170 (1997).
Bernstein, H. & Langer, R. Ex vivo model of an immobilized-enzyme reactor. Proc. Natl Acad. Sci. USA 85, 8751–8755 (1988).
Stroock, A. D. et al. Chaotic mixer for microchannels. Science 295, 647–651 (2002).
Rohlfing, C. L., Parvin, C. A., Sacks, D. B. & Little, R. R. Comparing analytic performance criteria: evaluation of HbA1c certification criteria as an example. Clin. Chim. Acta 433, 259–263 (2014).
Wong, D. L., Hockenberry-Eaton, M., Wilson, D., Winkelstein, M. L. & Schwartz, P. Wong’s Essentials of Pediatric Nursing (Mosby, 2001).
Berger, R. S. & Bowman, J. P. A reappraisal of the 21-day cumulative irritation test in man. J. Toxicol. Cutan. Ocul. Toxicol. 1, 109–115 (1982).
Mijailovic, A. S. et al. Optimizing outpatient phlebotomy staffing: tools to assess staffing needs and monitor effectiveness. Arch. Pathol. Lab. Med. 138, 929–935 (2014).
Zhong, J., Asker, C. L. & Salerud, E. G. Imaging, image processing and pattern analysis of skin capillary ensembles. Skin. Res. Technol. 6, 45–57 (2000).
Van Slyke, D. D. et al. Calculation of hemoglobin from bloodspecific gravities. J. Biol. Chem. 183, 349–360 (1949).
Klein, M. D., Drongowski, R. A., Linhardt, R. J. & Langer, R. S. A colorimetric assay for chemical heparin in plasma. Anal. Biochem. 124, 59–64 (1982).
Blicharz, T. M. et al. Dataset for Microneedle-based device for the one-step painless collection of capillary blood samples. figshare https://doi.org/10.6084/m9.figshare.c.3957568 (2017).
Acknowledgements
We thank M. Gilpatrick, M. Oster, and J. P. Lock for their clinical operations support during internal pilot testing. We also thank the principal investigators and clinical staff at each of the sites that supported the TAP external study: V. Bush (Mary Imogene Bassett Hospital), G. Allen (Roger Williams Medical Center), and Y. Henry (Geisinger Medical Center). We thank W. Fowle (Northeastern University) for providing the SEM image of the microneedle array. We also thank R. Langer for reviewing the manuscript. This work was funded in part by a grant from the Bill and Melinda Gates Foundation (OPP1028750—Point-of-Care Diagnostics).
Author information
Authors and Affiliations
Contributions
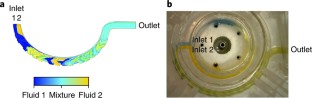
M.D. acquired the high-speed video of actuation mechanisms. C.A.T. analysed the high-speed video data. T.M.B. and P.G. designed and conducted the microneedle length study. R.E.W. conducted the Monte Carlo simulations. B.M.B. and K.M.L. designed and conducted the vacuum optimization and sampling site comparison studies. B.M.B. modelled and evaluated the microfluidic mixer. L.L.C. and R.K. designed and developed in vitro test systems for flowing liquids into the device. B.M.B. and R.K. performed the microfluidic mixing visualization study with coloured solutions. P.G. and S.L.M. designed and conducted the anticoagulant controlled-release studies. T.M.B. designed the TAP device clinical study. T.M.B, K.M.L., and J.A.W. collated and analysed data for the TAP device clinical study. T.M.B., B.M.B. and R.E.W. wrote and revised the paper. R.E.W. produced data analysis plots. S.P.D., R.H., D.E.C. and H.B. conceived of early microneedle-based blood collection device concepts and/or performed initial development work. All co-authors reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
T.M.B., P.G., B.M.B., L.L.C., K.M.L., J.A.W. and R.E.W. are employees of Seventh Sense Biosystems. The technology described in this paper is covered under the US patents 8,827,971 and 9,033,898.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Blicharz, T.M., Gong, P., Bunner, B.M. et al. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat Biomed Eng 2, 151–157 (2018). https://doi.org/10.1038/s41551-018-0194-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0194-1
This article is cited by
-
M2 macrophage-polarized anti-inflammatory microneedle patch for accelerating biofilm-infected diabetic wound healing via modulating the insulin pathway
Journal of Nanobiotechnology (2024)
-
Microneedle patch casting using a micromachined carbon master for enhanced drug delivery
Scientific Reports (2024)
-
The promise of microneedle technologies for drug delivery
Drug Delivery and Translational Research (2024)
-
Advances in microneedles for transdermal diagnostics and sensing applications
Microchimica Acta (2024)
-
Microneedle-based biosensing
Nature Reviews Bioengineering (2023)