Abstract
Microneedles, the miniaturized needles, which can pierce the skin with minimal invasiveness open up new possibilities for constructing personalized Point-of-Care (POC) diagnostic platforms. Recent advances in microneedle-based POC diagnostic systems, especially their successful implementation with wearable technologies, enable biochemical detection and physiological recordings in a user-friendly manner. This review presents an overview of the current advances in microneedle-based sensor devices, with emphasis on the biological basis of transdermal sensing, fabrication, and application of different types of microneedles, and a summary of microneedle devices based on various sensing strategies. It concludes with the challenges and future prospects of this swiftly growing field. The aim is to present a critical and thorough analysis of the state-of-the-art development of transdermal diagnostics and sensing devices based on microneedles, and to bridge the gap between microneedle technology and pragmatic applications.
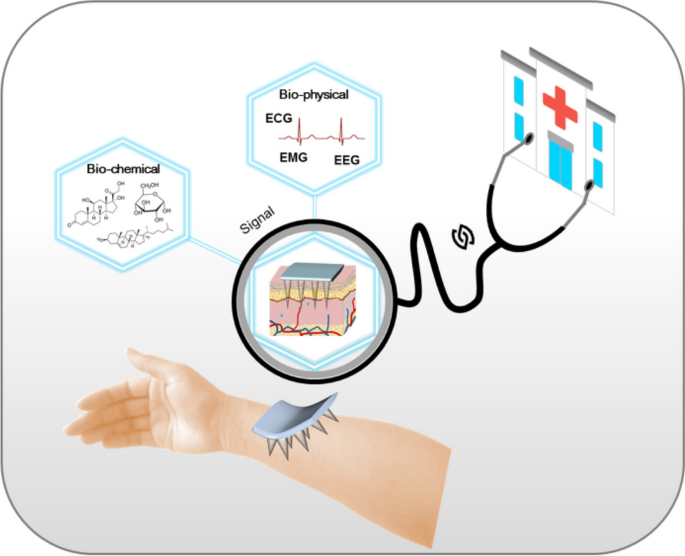
Graphical Abstract






Similar content being viewed by others
Data availability
Not applicable.
References
Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101:2087–2092
Hamilton JG (1995) Needle phobia: a neglected diagnosis. J Fam Pract 41:169
Hirobe S, Azukizawa H, Matsuo K, Zhai Y, Quan Y-S, Kamiyama F, Suzuki H, Katayama I, Okada N, Nakagawa S (2013) Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm Res 30:2664–2674
Top 10 Emerging Technologies (2020) World economic forum. https://www.weforum.org/publications/top-10-emergingtechnologies-2020/
Gerstel MS, Place VA (1976) Drug delivery device. Alza Corporation, Palo Alto
Smart WH, Subramanian K (2000) The use of silicon microfabrication technology in painless blood glucose monitoring. Diabetes Technol Ther 2:549–559
Gardeniers HJ, Luttge R, Berenschot EJ, De Boer MJ, Yeshurun SY, Hefetz M, Van't Oever R, Van Den Berg A (2003) Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst 12:855–862
Mukerjee EV, Collins SD, Isseroff RR, Smith RL (2004) Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens Actuator Phys 114:267–275
Sullivan SP, Koutsonanos DG, del Pilar Martin M, Lee JW, Zarnitsyn V, Choi S-O, Murthy N, Compans RW, Skountzou I, Prausnitz MR (2010) Dissolving polymer microneedle patches for influenza vaccination. Nat Med 16:915–920
Wang J, Shah D, Chen X, Anderson RR, Wu MX (2014) A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nat Commun 5:4447
Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA, Buse JB, Gu Z (2018) Core–Shell Microneedle Gel for Self-regulated insulin delivery. ACS Nano 12:2466–2473
Yu J, Wang J, Zhang Y, Chen G, Mao W, Ye Y, Kahkoska AR, Buse JB, Langer R, Gu Z (2020) Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng 4:499–506
Zhang Y, Liu Q, Yu J, Yu S, Wang J, Qiang L, Gu Z (2017) Locally Induced Adipose tissue Browning by Microneedle Patch for obesity treatment. ACS Nano 11:9223–9230
Yang G, Chen Q, Wen D, Chen Z, Wang J, Chen G, Wang Z, Zhang X, Zhang Y, Hu Q, Zhang L, Gu Z (2019) A therapeutic Microneedle Patch made from hair-derived keratin for promoting hair regrowth. ACS Nano 13:4354–4360
Lee K, Xue Y, Lee J, Kim H-J, Liu Y, Tebon P, Sarikhani E, Sun W, Zhang S, Haghniaz R, Çelebi-Saltik B, Zhou X, Ostrovidov S, Ahadian S, Ashammakhi N, Dokmeci MR, Khademhosseini A (2020) A patch of detachable hybrid microneedle depot for localized delivery of mesenchymal stem cells in regeneration therapy. Adv Funct Mater 30:2000086
Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z (2015) Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA 112:8260
Larrañeta E, McCrudden MTC, Courtenay AJ, Donnelly RF (2016) Microneedles: a new frontier in nanomedicine delivery. Pharm Res 33:1055–1073
Liu G-S, Kong Y, Wang Y, Luo Y, Fan X, Xie X, Yang B-R, Wu MX (2020) Microneedles for transdermal diagnostics: recent advances and new horizons. Biomaterials 232:119740
Ventrelli L, Marsilio Strambini L, Barillaro G (2015) Microneedles for Transdermal Biosensing: current picture and future direction. Adv Healthc Mater 4:2606–2640
García-Guzmán JJ, Pérez-Ràfols C, Cuartero M, Crespo GA (2021) Microneedle based electrochemical (Bio) sensing: towards decentralized and continuous health status monitoring. TrAC Trends Anal Chem 135:116148
Wang J, Lu Z, Cai R, Zheng H, Yu J, Zhang Y, Gu Z (2023) Microneedle-based transdermal detection and sensing devices. Lab Chip 23:869–887
Li J, Wei M, Gao B (2024) A review of recent advances in Microneedle-based sensing within the dermal ISF that could transform medical testing. ACS Sens 9:1149–1161
Teymourian H, Tehrani F, Mahato K, Wang J (2021) Lab under the skin: Microneedle Based Wearable devices. Adv Healthc Mater 10:2002255
Liu X, Kruger P, Maibach H, Colditz PB, Roberts MS (2014) Using skin for drug delivery and diagnosis in the critically ill. Adv Drug Delivery Rev 77:40–49
Luppa PB, Müller C, Schlichtiger A, Schlebusch H (2011) Point-of-care testing (POCT): current techniques and future perspectives. TrAC, Trends Anal Chem 30:887–898
Vora LK, Sabri AH, McKenna PE, Himawan A, Hutton ARJ, Detamornrat U, Paredes AJ, Larrañeta E, Donnelly RF (2023) Microneedle-based biosensing. Nat Rev Bioeng 2:64–81
Kolarsick PA, Kolarsick MA, Goodwin C (2011) Anatomy and physiology of the skin. J Dermatol Nurses’ Assoc 3:203–213
Lopes B, Sousa P, Alvites R, Branquinho M, Sousa A, Mendonça C, Atayde LM, Maurício AC (2021) The application of mesenchymal stem cells on wound repair and regeneration. Appl Sci 11:3000
Baroli B (2010) Penetration of nanoparticles and nanomaterials in the skin: fiction or reality? J Pharm Sci 99:21–50
Xue P, Zhang L, Xu Z, Yan J, Gu Z, Kang Y (2018) Blood sampling using microneedles as a minimally invasive platform for biomedical diagnostics. Appl Mater Today 13:144–157
Bouwstra JA, Gooris GS, van der Spek JA, Bras W (1991) Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol 97:1005–1012
Bal SM, Ding Z, van Riet E, Jiskoot W, Bouwstra JA (2010) Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release 148:266–282
Wong R, Geyer S, Weninger W, Guimberteau J-C, Wong JK (2016) The dynamic anatomy and patterning of skin. Exp Dermatol 25:92–98
Woo WM (2019) Skin structure and biology. In: Xu C, Wang X, Pramanik M (eds) Imaging technologies and transdermal delivery in skin disorders. https://doi.org/10.1002/9783527814633.ch1
Lowe JS, Anderson PG (2015) Chap. 18-skin and breast. In: Lowe JS, Anderson PG (eds) Stevens & Lowe’s human histology, 4th edn. Mosby, Philadelphia, pp 363–384
Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz Á (2016) Chap. 1-anatomy and function of the skin. In: Hamblin MR, Avci P, Prow TW (eds) Nanoscience in Dermatology. Academic Press, Boston, pp 1–14
Samant PP, Niedzwiecki MM, Raviele N, Tran V, Mena-Lapaix J, Walker DI, Felner EI, Jones DP, Miller GW, Prausnitz MR (2020) Sampling interstitial fluid from human skin using a microneedle patch. Sci Transl Med 12:eaaw0285
Roe JN, Smoller BR (1998) Bloodless glucose measurements. Crit Rev Ther Drug Carr Syst 15:199–241
Niedzwiecki MM, Samant P, Walker DI, Tran V, Jones DP, Prausnitz MR, Miller GW (2018) Human suction blister fluid composition determined using high-resolution metabolomics. Anal Chem 90:3786–3792
Strambini LM, Longo A, Scarano S, Prescimone T, Palchetti I, Minunni M, Giannessi D, Barillaro G (2015) Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens Bioelectron 66:162–168
Nilsson AK, Sjöbom U, Christenson K, Hellström A (2019) Lipid profiling of suction blister fluid: comparison of lipids in interstitial fluid and plasma. Lipids Health Dis 18:164
Koschinsky T, Jungheim K, Heinemann L (2003) Glucose sensors and the alternate site testing-like phenomenon: relationship between rapid blood glucose changes and glucose sensor signals. Diabetes Technol Ther 5:829–842
Fogh-Andersen N, Altura BM, Altura BT, Siggaard-Andersen O (1995) Composition of interstitial fluid. Clin Chem 41:1522–1525
Marunaka Y (2015) Roles of interstitial fluid pH in diabetes mellitus: Glycolysis and mitochondrial function. World J Diabetes 6:125–135
Zhu DD, Zheng LW, Duong PK, Cheah RH, Liu XY, Wong JR, Wang WJ, Tien Guan ST, Zheng XT, Chen P (2022) Colorimetric microneedle patches for multiplexed transdermal detection of metabolites. Biosens Bioelectron 212:114412
Wu Z, Qiao Z, Chen S, Fan S, Liu Y, Qi J, Lim CT (2024) Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications. Commun Mater 5:33
Friedel M, Thompson IAP, Kasting G, Polsky R, Cunningham D, Soh HT, Heikenfeld J (2023) Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat Biomed Eng 7:1541–1555
Krogstad AL, Jansson P-A, Gisslén P, Lönnroth P (1996) Microdialysis methodology for the measurement of dermal interstitial fluid in humans. Br J Dermatol 134:1005–1012
Wang Y, Wu Y, Lei Y (2023) Microneedle-based glucose monitoring: a review from sampling methods to wearable biosensors. Biomater Sci 11:5727–5757
Sang M, Cho M, Lim S, Min IS, Han Y, Lee C, Shin J, Yoon K, Yeo W-H, Lee T, Won SM, Jung Y, Heo YJ, Yu KJ (2023) Fluorescent-based biodegradable microneedle sensor array for tether-free continuous glucose monitoring with smartphone application. Sci Adv 9:eadh1765
Strindberg L, Lönnroth P (2000) Validation of an endogenous reference technique for the calibration of microdialysis catheters. Scand J Clin Lab Invest 60:205–212
Saifullah KM, Faraji Rad Z (2023) Sampling dermal interstitial fluid using microneedles: a review of recent developments in sampling methods and microneedle-based biosensors. Adv Mater Interfaces 10:2201763
Caliò A, Dardano P, Di Palma V, Bevilacqua MF, Di Matteo A, Iuele H, De Stefano L (2016) Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens Actuators B Chem 236:343–349
Maggs DG, Jacob R, Rife F, Lange R, Leone P, During MJ, Tamborlane WV, Sherwin RS (1995) Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadricep muscle and adipose tissue. Evidence for significant lipolysis in skeletal muscle. J Clin Invest 96:370–377
Blicharz TM, Gong P, Bunner BM, Chu LL, Leonard KM, Wakefield JA, Williams RE, Dadgar M, Tagliabue CA, El Khaja R, Marlin SL, Haghgooie R, Davis SP, Chickering DE, Bernstein H (2018) Microneedle-based device for the one-step painless collection of capillary blood samples. Nat Biomed Eng 2:151–157
Wang Q, Molinero-Fernandez A, Casanova A, Titulaer J, Campillo-Brocal JC, Konradsson-Geuken Å, Crespo GA, Cuartero M (2022) Intradermal glycine detection with a wearable microneedle biosensor: the first in vivo assay. Anal Chem 94:11856–11864
Liu H, Shao J, Shi L, Ke W, Zheng F, Zhao Y (2020) Electroactive NPs and D-amino acids oxidase engineered electrochemical chiral sensor for D-alanine detection. Sens Actuators B Chem 304:127333
Mackness MI, Mackness B, Arrol S, Wood G, Bhatnagar D, Durrington PN (1997) Presence of paraoxonase in human interstitial fluid. FEBS Lett 416:377–380
Narwal V, Deswal R, Batra B, Kalra V, Hooda R, Sharma M, Rana JS (2019) Cholesterol biosensors: a review. Steroids 143:6–17
Chang K-T, Shen Y-K, Fan F-Y, Lin Y, Kang S-C (2020) Optimal design and fabrication of a microneedle arrays patch. J Manuf Process 54:274–285
Kochhar JS, Quek TC, Soon WJ, Choi J, Zou S, Kang L (2013) Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J Pharm Sci 102:4100–4108
Crichton ML, Chen X, Huang H, Kendall MAF (2013) Elastic modulus and viscoelastic properties of full thickness skin characterised at micro scales. Biomaterials 34:2087–2097
Kendall MAF, Chong Y-F, Cock A (2007) The mechanical properties of the skin epidermis in relation to targeted gene and drug delivery. Biomaterials 28:4968–4977
Pailler-Mattei C, Bec S, Zahouani H (2008) In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys 30:599–606
Chua B, Desai SP, Tierney MJ, Tamada JA, Jina AN (2013) Effect of microneedles shape on skin penetration and minimally invasive continuous glucose monitoring in vivo. Sens Actuator Phys 203:373–381
Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF (2016) Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep 104:1–32
Lee kJ, Jeong S, Roh D, Kim D, Choi H-K, Lee E (2019) A practical guide to the development of microneedle systems–in clinical trials or on the market. Int J Pharm 573:118778
Aldawood FK, Andar A, Desai S (2021) A comprehensive review of microneedles: types, materials, processes, characterizations and applications. Polymers 13:2815
Kolluru C, Williams M, Chae J, Prausnitz MR (2019) Recruitment and Collection of dermal interstitial fluid using a Microneedle Patch. Adv Healthc Mater 8:1801262
Wang PM, Cornwell M, Prausnitz MR (2005) Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther 7:131–141
Chang H, Zheng M, Yu X, Than A, Seeni RZ, Kang R, Tian J, Khanh DP, Liu L, Chen P, Xu C (2017) A Swellable Microneedle Patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv Mater 29:1702243
Al Sulaiman D, Chang JYH, Bennett NR, Topouzi H, Higgins CA, Irvine DJ, Ladame S (2019) Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano 13:9620–9628
Romanyuk AV, Zvezdin VN, Samant P, Grenader MI, Zemlyanova M, Prausnitz MR (2014) Collection of analytes from Microneedle Patches. Anal Chem 86:10520–10523
Li CG, Dangol M, Lee CY, Jang M, Jung H (2015) A self-powered one-touch blood extraction system: a novel polymer-capped hollow microneedle integrated with a pre-vacuum actuator. Lab Chip 15:382–390
Liu L, Wang Y, Yao J, Yang C, Ding G (2016) A minimally invasive micro sampler for quantitative sampling with an ultrahigh-aspect-ratio microneedle and a PDMS actuator. Biomed Microdevices 18:59
Corrie SR, Fernando GJP, Crichton ML, Brunck MEG, Anderson CD, Kendall MAF (2010) Surface-modified microprojection arrays for intradermal biomarker capture, with low non-specific protein binding. Lab Chip 10:2655–2658
Bhargav A, Muller DA, Kendall MAF, Corrie SR (2012) Surface modifications of microprojection arrays for Improved Biomarker capture in the skin of live mice. ACS Appl Mater Interfaces 4:2483–2489
Muller DA, Corrie SR, Coffey J, Young PR, Kendall MA (2012) Surface modified microprojection arrays for the selective extraction of the Dengue Virus NS1 protein as a marker for disease. Anal Chem 84:3262–3268
Coffey JW, Meliga SC, Corrie SR, Kendall MAF (2016) Dynamic application of microprojection arrays to skin induces circulating protein extravasation for enhanced biomarker capture and detection. Biomaterials 84:130–143
Coffey JW, Corrie SR, Kendall MAF (2018) Rapid and selective sampling of IgG from skin in less than 1 min using a high surface area wearable immunoassay patch. Biomaterials 170:49–57
Lee JW, Park J-H, Prausnitz MR (2008) Dissolving microneedles for transdermal drug delivery. Biomaterials 29:2113–2124
Caffarel-Salvador E, Brady AJ, Eltayib E, Meng T, Alonso-Vicente A, Gonzalez-Vazquez P, Torrisi BM, Vicente-Perez EM, Mooney K, Jones DS, Bell SEJ, McCoy CP, McCarthy HO, McElnay JC, Donnelly RF (2015) Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PLoS ONE 10:e0145644–e0145644
Eltayib E, Brady AJ, Caffarel-Salvador E, Gonzalez-Vazquez P, Zaid Alkilani A, McCarthy HO, McElnay JC, Donnelly RF (2016) Hydrogel-forming microneedle arrays: potential for use in minimally-invasive lithium monitoring. Eur J Pharm Biopharm 102:123–131
Yeow B, Coffey JW, Muller DA, Grøndahl L, Kendall MAF, Corrie SR (2013) Surface modification and characterization of Polycarbonate Microdevices for capture of circulating biomarkers, both in Vitro and in vivo. Anal Chem 85:10196–10204
Bao L, Park J, Bonfante G, Kim B (2022) Recent advances in porous microneedles: materials, fabrication, and transdermal applications. Drug Deliv Transl Res 12:395–414
Yi K, Wang Y, Shi K, Chi J, Lyu J, Zhao Y (2021) Aptamer-decorated porous microneedles arrays for extraction and detection of skin interstitial fluid biomarkers. Biosens Bioelectron 190:113404
Lee H, Bonfante G, Sasaki Y, Takama N, Minami T, Kim B (2020) Porous microneedles on a paper for screening test of prediabetes. Med Devices Sens 3:e10109
Pang Y, Li Y, Chen K, Wu M, Zhang J, Sun Y, Xu Y, Wang X, Wang Q, Ning X, Kong D (2024) Porous microneedles through direct ink drawing with nanocomposite inks for transdermal collection of interstitial fluid. Small 20:2305838
Sartawi Z, Blackshields C, Faisal W (2022) Dissolving microneedles: applications and growing therapeutic potential. J Control Release 348:186–205
Li S, Kim Y, Lee JW, Prausnitz MR (2022) Microneedle patch tattoos. iScience 25:105014
He R, Liu H, Fang T, Niu Y, Zhang H, Han F, Gao B, Li F, Xu F (2021) A colorimetric dermal tattoo biosensor fabricated by microneedle patch for multiplexed detection of health-related biomarkers. Adv Sci 8:2103030
Yetisen AK, Moreddu R, Seifi S, Jiang N, Vega K, Dong X, Dong J, Butt H, Jakobi M, Elsner M, Koch AW (2019) Dermal tattoo biosensors for colorimetric metabolite detection. Angew Chem Int Ed 58:10506–10513
Chakraborty S, Tsuchiya K (2008) Development and fluidic simulation of microneedles for painless pathological interfacing with living systems. J Appl Phys 103:114701
Li CG, Lee K, Lee CY, Dangol M, Jung H (2012) A minimally invasive blood-extraction system: Elastic Self-Recovery Actuator Integrated with an Ultrahigh- Aspect-Ratio Microneedle. Adv Mater 24:4583–4586
Paik S-J, Byun S, Lim J-M, Park Y, Lee A, Chung S, Chang J, Chun K, Cho DD (2004) In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens Actuator Phys 114:276–284
Xie Y, He J, He W, Iftikhar T, Zhang C, Su L, Zhang X (2024) Enhanced interstitial fluid extraction and rapid analysis via vacuum tube-integrated microneedle array device. Adv Sci 11:2308716
Li T, Barnett A, Rogers KL, Gianchandani YB (2009) A blood sampling microsystem for pharmacokinetic applications: design, fabrication, and initial results. Lab Chip 9:3495–3503
Samant PP, Prausnitz MR (2018) Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. U.S.A 115:4583–4588
Zheng M, Wang Z, Chang H, Wang L, Chew SWT, Lio DCS, Cui M, Liu L, Tee BCK, Xu C (2020) Osmosis-powered hydrogel microneedles for microliters of skin interstitial fluid extraction within Minutes. Adv Healthc Mater 9:1901683
Mishra RK, Goud KY, Li Z, Moonla C, Mohamed MA, Tehrani F, Teymourian H, Wang J (2020) Continuous opioid monitoring along with nerve agents on a wearable microneedle sensor array. J Am Chem Soc 142:5991–5995
Tehrani F, Teymourian H, Wuerstle B, Kavner J, Patel R, Furmidge A, Aghavali R, Hosseini-Toudeshki H, Brown C, Zhang F, Mahato K, Li Z, Barfidokht A, Yin L, Warren P, Huang N, Patel Z, Mercier PP, Wang J (2022) An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat Biomed Eng 6:1214–1224
Liu F, Lin Z, Jin Q, Wu Q, Yang C, Chen H-J, Cao Z, Lin D-a, Zhou L, Hang T, He G, Xu Y, Xia W, Tao J, Xie X (2019) Protection of nanostructures-integrated microneedle biosensor using dissolvable polymer coating. ACS Appl Mater Interfaces 11:4809–4819
Zhang P, Wu X, Xue H, Wang Y, Luo X, Wang L (2022) Wearable transdermal colorimetric microneedle patch for uric acid monitoring based on peroxidase-like polypyrrole nanoparticles. Anal Chim Acta 1212:339911
Li CG, Joung H-A, Noh H, Song M-B, Kim M-G, Jung H (2015) One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip 15:3286–3292
Goud KY, Moonla C, Mishra RK, Yu C, Narayan R, Litvan I, Wang J (2019) Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens 4:2196–2204
Wang Z, Li H, Wang J, Chen Z, Chen G, Wen D, Chan A, Gu Z (2020) Transdermal colorimetric patch for hyperglycemia sensing in diabetic mice. Biomaterials 237:119782
Cheng Y, Gong X, Yang J, Zheng G, Zheng Y, Li Y, Xu Y, Nie G, Xie X, Chen M, Yi C, Jiang L (2022) A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens Bioelectron 203:114026
Joshi P, Riley PR, Mishra R, Azizi Machekposhti S, Narayan R (2022) Transdermal polymeric microneedle sensing platform for fentanyl detection in biofluid. Biosensors 12:198
Paul R, Ostermann E, Chen Y, Saville AC, Yang Y, Gu Z, Whitfield AE, Ristaino JB, Wei Q (2021) Integrated microneedle-smartphone nucleic acid amplification platform for in-field diagnosis of plant diseases. Biosens Bioelectron 187:113312
Ciui B, Martin A, Mishra RK, Brunetti B, Nakagawa T, Dawkins TJ, Lyu M, Cristea C, Sandulescu R, Wang J (2018) Wearable wireless tyrosinase bandage and microneedle sensors: toward melanoma screening. Adv Healthc Mater 7:1701264
Yoon Y, Lee GS, Yoo K, Lee J-B (2013) Fabrication of a microneedle/CNT hierarchical micro/nano surface electrochemical sensor and its in-vitro glucose sensing characterization. Sensors 13:16672–16681
McConville A, Davis J (2016) Transdermal microneedle sensor arrays based on palladium: polymer composites. Electrochem Commun 72:162–165
Chen K, Ren L, Chen Z, Pan C, Zhou W, Jiang L (2016) Fabrication of micro-needle electrodes for bio-signal recording by a magnetization-induced self-assembly method. Sensors 16:1533
Zuliani C, Ng FS, Alenda A, Eftekhar A, Peters NS, Toumazou C (2016) An array of individually addressable micro-needles for mapping pH distributions. Analyst 141:4659–4666
Invernale MA, Tang BC, York RL, Le L, Hou DY, Anderson DG (2014) Microneedle electrodes toward an amperometric glucose-sensing Smart Patch. Adv Healthc Mater 3:338–342
Mohan AMV, Windmiller JR, Mishra RK, Wang J (2017) Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens Bioelectron 91:574–579
Chen D, Wang C, Chen W, Chen Y, Zhang JXJ (2015) PVDF-Nafion nanomembranes coated microneedles for in vivo transcutaneous implantable glucose sensing. Biosens Bioelectron 74:1047–1052
Bollella P, Sharma S, Cass AEG, Antiochia R (2019) Microneedle-based biosensor for minimally-invasive lactate detection. Biosens Bioelectron 123:152–159
Chinnadayyala SR, Park I, Cho S (2018) Nonenzymatic determination of glucose at near neutral pH values based on the use of nafion and platinum black coated microneedle electrode array. Mikrochim Acta 185:250
Shervedani RK, Karevan M, Amini A (2014) Prickly nickel nanowires grown on Cu substrate as a supersensitive enzyme-free electrochemical glucose sensor. Sens Actuators B Chem 204:783–790
Lee SJ, Yoon HS, Xuan X, Park JY, Paik S-J, Allen MG (2016) A patch type non-enzymatic biosensor based on 3D SUS micro-needle electrode array for minimally invasive continuous glucose monitoring. Sens Actuators B Chem 222:1144–1151
Windmiller JR, Zhou N, Chuang M-C, Valdés-Ramírez G, Santhosh P, Miller PR, Narayan R, Wang J (2011) Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 136:1846–1851
Windmiller JR, Valdés-Ramírez G, Zhou N, Zhou M, Miller PR, Jin C, Brozik SM, Polsky R, Katz E, Narayan R, Wang J (2011) Bicomponent microneedle array Biosensor for minimally-invasive glutamate monitoring. Electroanalysis 23:2302–2309
Miller PR, Xiao X, Brener I, Burckel DB, Narayan R, Polsky R (2014) Microneedle-based transdermal sensor for on-chip potentiometric determination of K+. Adv Healthc Mater 3:876–881
Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, Goldman SA, Bredel M, McLendon RE, Sloan AE, Hjelmeland AB, Rich JN (2011) Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146:53–66
Keum DH, Jung HS, Wang T, Shin MH, Kim Y-E, Kim KH, Ahn G-O, Hahn SK (2015) Microneedle biosensor for real-time electrical detection of nitric oxide for in situ cancer diagnosis during endomicroscopy. Adv Healthc Mater 4:1153–1158
Gao J, Huang W, Chen Z, Yi C, Jiang L (2019) Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens Actuators B Chem 287:102–110
Teymourian H, Moonla C, Tehrani F, Vargas E, Aghavali R, Barfidokht A, Tangkuaram T, Mercier PP, Dassau E, Wang J (2019) Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal Chem 92:2291–2300
Lin Y, Zhao M, Guo Y, Ma X, Luo F, Guo L, Qiu B, Chen G, Lin Z (2016) Multicolor colormetric biosensor for the determination of glucose based on the etching of gold nanorods. Sci Rep 6:37879
Zhang P-P, Zhu J-C, Zhao B-J, Xu S-H, Wang L, Luo X-L (2022) Wearable transdermal microneedle patch based on photonic crystal hydrogel for glucose monitoring. Chin J Anal Chem 50:100054
Bao L, Park J, Qin B, Kim B (2022) Anti-SARS-CoV-2 IgM/IgG antibodies detection using a patch sensor containing porous microneedles and a paper-based immunoassay. Sci Rep 12:10693
Kim D, Cao Y, Mariappan D, Bono MS Jr, Hart AJ, Marelli B (2021) A microneedle technology for sampling and sensing bacteria in the food supply chain. Adv Funct Mater 31:2005370
Jina A, Tierney MJ, Tamada JA, McGill S, Desai S, Chua B, Chang A, Christiansen M (2014) Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J Diabetes Sci Technol 8:483–487
Nicholas D, Logan KA, Sheng Y, Gao J, Farrell S, Dixon D, Callan B, McHale AP, Callan JF (2018) Rapid paper based colorimetric detection of glucose using a hollow microneedle device. Int J Pharm 547:244–249
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 48:1004–1076
Liao J, Ye C, Agrawal P, Gu Z, Zhang YS (2021) Colloidal photonic crystals for Biomedical Applications. Small Struct 2:2000110
Zeng Y, Wang J, Wang Z, Chen G, Yu J, Li S, Li Q, Li H, Wen D, Gu Z, Gu Z (2020) Colloidal crystal microneedle patch for glucose monitoring. Nano Today 35:100984
Zhang X, Chen G, Bian F, Cai L, Zhao Y (2019) Encoded microneedle arrays for detection of skin interstitial fluid biomarkers. Adv Mater 31:1902825
Park JE, Yonet-Tanyeri N, Vander Ende E, Henry A-I, Perez White BE, Mrksich M, Van Duyne RP (2019) Plasmonic microneedle arrays for in situ sensing with surface-enhanced Raman spectroscopy (SERS). Nano Lett 19:6862–6868
Ju J, Hsieh C-M, Tian Y, Kang J, Chia R, Chang H, Bai Y, Xu C, Wang X, Liu Q (2020) Surface enhanced Raman spectroscopy based biosensor with a microneedle array for minimally invasive in vivo glucose measurements. ACS Sensors 5:1777–1785
Wang Z, Luan J, Seth A, Liu L, You M, Gupta P, Rathi P, Wang Y, Cao S, Jiang Q, Zhang X, Gupta R, Zhou Q, Morrissey JJ, Scheller EL, Rudra JS, Singamaneni S (2021) Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat Biomed Eng 5:64–76
Keyvani F, Zheng H, Kaysir MR, Mantaila DF, Ghavami Nejad P, Rahman FA, Quadrilatero J, Ban D, Poudineh M (2023) A hydrogel microneedle assay combined with nucleic acid probes for on-site detection of small molecules and proteins. Angew Chem Int Ed 135:e202301624
Lee KT, Muller DA, Coffey JW, Robinson KJ, McCarthy JS, Kendall MAF, Corrie SR (2014) Capture of the circulating plasmodium falciparum biomarker HRP2 in a multiplexed format, via a wearable skin patch. Anal Chem 86:10474–10483
Coffey JW, Corrie SR, Kendall MAF (2013) Early circulating biomarker detection using a wearable microprojection array skin patch. Biomaterials 34:9572–9583
Ling Y, An T, Yap LW, Zhu B, Gong S, Cheng W (2020) Disruptive, soft, wearable sensors. Adv Mater 32:1904664
Li J, Ma Y, Huang D, Wang Z, Zhang Z, Ren Y, Hong M, Chen Y, Li T, Shi X, Cao L, Zhang J, Jiao B, Liu J, Sun H, Li Z (2022) High-performance flexible microneedle array as a low-impedance surface biopotential dry electrode for wearable electrophysiological recording and polysomnography. Nano-Micro Lett 14:132
Forvi E, Bedoni M, Carabalona R, Soncini M, Mazzoleni P, Rizzo F, O’Mahony C, Morasso C, Cassarà DG, Gramatica F (2012) Preliminary technological assessment of microneedles-based dry electrodes for biopotential monitoring in clinical examinations. Sens Actuator A Phys 180:177–186
Wang R, Jiang X, Wang W, Li Z (2017) A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sens Actuators B Chem 244:750–758
Kim M, Gu G, Cha K, Kim D, Chung W (2017) Wireless sEMG system with a microneedle-based high-density electrode array on a flexible substrate. Sensors 18:92
Searle A, Kirkup L (2000) A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol Meas 21:271–283
Griss P, Enoksson P, Tolvanen-Laakso HK, Merilainen P, Ollmar S, Stemme G (2001) Micromachined electrodes for biopotential measurements. J Microelectromech Syst 10:10–16
Kim M, Kim T, Kim DS, Chung WK (2015) Curved microneedle array-based sEMG electrode for Robust Long-Term measurements and high selectivity. Sensors 15:16265–16280
Patrick G, Enoksson P (2002) Micromachined barbed spikes for mechanical chip attachment. Sens Actuator A Phys 95:94–99
Pei W, Zhang H, Wang Y, Guo X, Xing X, Huang Y, Xie Y, Yang X, Chen H (2017) Skin-potential variation insensitive dry electrodes for ECG recording. IEEE Trans Biomed Eng 64:463–470
Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR (2014) Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine 32:1856–1862
Acknowledgements
This paper is dedicated to the memory of Otto S. Wolfbeis. This work is supported by the National Natural Science Foundation of China (22174082, 22374085), the Key Research and Development Program of Shandong Province (2021ZDSYS30), and the Science and Technology Benefiting the People Project of Qingdao (20-3-4-53-nsh).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This work did not involve human or animal samples.
Competing interests
Xiliang Luo is an editor of this journal and recused himself from all decisions about this paper. Otherwise, all authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Wang, Y., Wu, X. et al. Advances in microneedles for transdermal diagnostics and sensing applications. Microchim Acta 191, 406 (2024). https://doi.org/10.1007/s00604-024-06458-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06458-2



