Abstract
Backgrounds
With an increase in the world’s aging population, candidate substances for the management of neurodegenerative diseases, including Alzheimer’s disease (AD), have received considerable attention. Amyloid beta (Aβ) peptide, the leading cause of AD, induces synaptic deficits, eventually leading to cognitive impairment. Plasmalogen is a subclass of glycerophospholipids containing a vinyl ether group in the glycerol backbone which has various efficacies.
Objectives
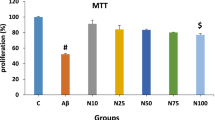
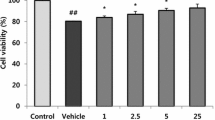
In this study, we investigated the mechanisms of scallop-derived plasmalogen (SPL) in neurotoxicity in human neurons, which remain incompletely understood. SH-SY5Y human neuroblastaoma cells were treated with Aβ 1–42, a major component of amyloid deposits in AD brains.
Results
SPL inhibited the phosphorylation of glycogen synthase kinase 3β and tau induced by Aβ. In addition, SPL decreased Aβ generation by downregulating the expression of amyloid precursor protein (APP) and beta-site APP cleaving enzyme 1. Furthermore, SPL suppressed the release of pro-inflammatory cytokines and expression of apoptotic proteins induced by Aβ.
Conclusion
Our study indicates that SPL would reduce Aβ-induced neurotoxicity associated with inflammation and apoptosis in human neuroblastoma cells.





Similar content being viewed by others
Data availability
All data and results of the current study are available from the corresponding authors upon reasonable request.
References
Aktas O, Schulze-Topphoff U, Zipp F (2007) The role of TRAIL/TRAIL receptors in central nervous system pathology. Front Biosci Landmark 12(8):2912–2921. https://doi.org/10.2741/2281
Ali F, Hossain MS, Sejimo S, Akashi K (2019) Plasmalogens inhibit endocytosis of toll-like receptor 4 to attenuate the inflammatory signal in microglial cells. Mol Neurobiol 56:3404–3419. https://doi.org/10.1007/s12035-018-1307-2
Apelt J, Schliebs R (2001) β-amyloid-induced glial expression of both pro-and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res 894(1):21–30. https://doi.org/10.1016/s0006-8993(00)03176-0
Bennett SA et al (2013) Using neurolipidomics to identify phospholipid mediators of synaptic (dys) function in Alzheimer’s disease. Front Physiol 4:168. https://doi.org/10.3389/fphys.2013.00168
Che H et al (2018) EPA enriched ethanolamine plasmalogens significantly improve cognition of Alzheimer’s disease mouse model by suppressing β-amyloid generation. J Funct Foods 41:9–18. https://doi.org/10.1016/j.jff.2017.12.016
Cheng X, Shen Y, Li R (2014) Targeting TNF: a therapeutic strategy for Alzheimer’s disease. Drug Discovery Today 19(11):1822–1827. https://doi.org/10.1016/j.drudis.2014.06.029
Chipuk JE et al (2010) The BCL-2 family reunion. Mol Cell 37(3):299–310. https://doi.org/10.1016/j.molcel.2010.01.025
Constantinescu R, Constantinescu A, Reichmann H, Janetzky B (2007) Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. Springer. https://doi.org/10.1007/978-3-211-73574-9_3
Crowley LC, Marfell BJ, Waterhouse NJ (2016) Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harbor Protoc 9:87205. https://doi.org/10.1101/pdb.prot087205
de Medeiros LM et al (2019) Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s disease studies. Mol Neurobiol 56:7355–7367. https://doi.org/10.1007/s12035-019-1605-3
Feng J et al (2021) Protect effects of seafood-derived plasmalogens against amyloid-beta (1–42) induced toxicity via modulating the transcripts related to endocytosis, autophagy, apoptosis, neurotransmitter release and synaptic transmission in sh-sy5y cells. Front Aging Neurosci 13:773713. https://doi.org/10.3389/fnagi.2021.773713
Fricker M et al (2018) Neuronal cell death. Physiol Rev 98(2):813–880. https://doi.org/10.1152/physrev.00011.2017
Gardiner J, Overall R, Marc J (2011) The microtubule cytoskeleton acts as a key downstream effector of neurotransmitter signaling. Synapse 65(3):249–256. https://doi.org/10.1002/syn.20841
Goldie BJ, Barnett MM, Cairns MJ (2014) BDNF and the maturation of posttranscriptional regulatory networks in human SH-SY5Y neuroblast differentiation. Front Cell Neurosci 8:325. https://doi.org/10.3389/fncel.2014.00325
Han X, Holtzman DM, McKeel DW Jr (2001) Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem 77(4):1168–1180. https://doi.org/10.1046/j.1471-4159.2001.00332.x
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155. https://doi.org/10.1002/glia.10161
Hernández F, Avila J (2008) The role of glycogen synthase kinase 3 in the early stages of Alzheimers’ disease. FEBS Lett 582(28):3848–3854. https://doi.org/10.1016/j.febslet.2008.10.026
Hollville E, Romero SE, Deshmukh M (2019) Apoptotic cell death regulation in neurons. FEBS J 286(17):3276–3298. https://doi.org/10.1111/febs.14970
Hossain MS et al (2013) Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS ONE 8(12):e83508. https://doi.org/10.1371/journal.pone.0083508
Jones K, Kim DW, Park JS, Khang CH (2016) Live-cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae. BMC Plant Biol 16:1–8. https://doi.org/10.1186/s12870-016-0756-x
Kadokawa H et al (2022) Ethanolamine plasmalogens derived from scallops stimulate both follicle-stimulating hormone and luteinizing hormone secretion by bovine gonadotrophs. Sci Rep 12(1):16789. https://doi.org/10.1038/s41598-022-20794-4
Katafuchi T et al (2012) Effects of plasmalogens on systemic lipopolysaccharide-induced glial activation and β-amyloid accumulation in adult mice. Ann N Y Acad Sci 1262(1):85–92. https://doi.org/10.1111/j.1749-6632.2012.06641.x
Krause DL (2010) Müller N (2010) Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer’s disease. Int J Alzheimer’s Dis 2:2
Lebel M et al (2009) Dopamine D1 receptor activation induces tau phosphorylation via cdk5 and GSK3 signaling pathways. Neuropharmacology 57(4):392–402. https://doi.org/10.1016/j.neuropharm.2009.06.041
Lee Y-J et al (2010) Inflammation and Alzheimer’s disease. Arch Pharmacal Res 33:1539–1556. https://doi.org/10.1007/s12272-010-1006-7
Liu Y et al (2021) Plasmalogen attenuates the development of hepatic steatosis and cognitive deficit through mechanism involving p75NTR inhibition. Redox Biol 43:102002. https://doi.org/10.1016/j.redox.2021.102002
Martínez M-A et al (2020) Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ Int 135:105414. https://doi.org/10.1016/j.envint.2019.105414
Näslund J et al (2000) Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 283(12):1571–1577. https://doi.org/10.1001/jama.283.12.1571
Ng A et al (2018) IL-1β, IL-6, TNF-α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep 8(1):12050. https://doi.org/10.1038/s41598-018-30487-6
O’brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Ann Rev Neurosci 34:185–204. https://doi.org/10.1146/annurev-neuro-061010-113613
Patel NS et al (2005) Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J Neuroinflamm 2:1–10. https://doi.org/10.1186/1742-2094-2-9
Scheuner D et al (1996) Secreted amyloid β–protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 2(8):864–870. https://doi.org/10.1038/nm0896-864
Sejimo S, Hossain MS, Akashi K (2018) Scallop-derived plasmalogens attenuate the activation of PKCδ associated with the brain inflammation. Biochem Biophys Res Commun 503(2):837–842. https://doi.org/10.1016/j.bbrc.2018.06.084
Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399(6738):A23–A31. https://doi.org/10.1038/399a023
Selkoe DJ (2001) Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid\beta-protein. J Alzheimers Dis 3(1):75–82. https://doi.org/10.3233/jad-2001-3111
Tang Z et al (1853) (2015) mTor mediates tau localization and secretion: implication for Alzheimer’s disease. Biochim Biophys Acta Mol Cell Res 7:1646–1657. https://doi.org/10.1016/j.bbamcr.2015.03.003
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9(3):231–241. https://doi.org/10.1038/nrm2312
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267(5203):1456–1462. https://doi.org/10.1126/science.7878464
Tuppo EE, Arias HR (2005) The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol 37(2):289–305. https://doi.org/10.1016/j.biocel.2004.07.009
Wang W-Y, Tan M-S, Yu J-T, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Wang C-C et al (2019) Comparative studies of DHA-enriched phosphatidylcholine and recombination of DHA-ethyl ester with egg phosphatidylcholine on ameliorating memory and cognitive deficiency in SAMP8 mice. Food Funct 10(2):938–950. https://doi.org/10.1039/c8fo01822g
Wood PL et al (2010) Circulating plasmalogen levels and Alzheimer disease assessment scale–cognitive scores in Alzheimer patients. J Psychiatry Neurosci 35(1):59–62. https://doi.org/10.1503/jpn.090059
Woodroofe MN (1995) Cytokine production in the central nervous system. Neurology 45(6 Suppl 6):S6–S10. https://doi.org/10.1212/wnl.45.6_suppl_6.s6
Wu C-H et al (2017) Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase reduces brain damage and attenuates neuroinflammation after intracerebral hemorrhage. J Neuroinflammation 14:1–21. https://doi.org/10.1186/s12974-017-1005-4
Yang T-X et al (2022) Epa-enriched plasmalogen attenuates the cytotoxic effects of lps-stimulated microglia on the sh-sy5y neuronal cell line. Brain Res Bull. https://doi.org/10.1016/j.brainresbull.2022.06.002
Youssef M, Ibrahim A, Akashi K, Hossain MS (2019) PUFA-plasmalogens attenuate the LPS-induced nitric oxide production by inhibiting the NF-kB, p38 MAPK and JNK pathways in microglial cells. Neuroscience 397:18–30. https://doi.org/10.1016/j.neuroscience.2018.11.030
Zhang Y-P et al (2018) DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostagland Leukot Essent Fatty Acids 136:85–94. https://doi.org/10.1016/j.plefa.2017.07.003
Zhu Y, Ahlemeyer B, Bauerbach E, Krieglstein J (2001) TGF-β1 inhibits caspase-3 activation and neuronal apoptosis in rat hippocampal cultures. Neurochem Int 38(3):227–235. https://doi.org/10.1016/s0197-0186(00)00084-x
Acknowledgements
The authors gratefully acknowledge the support provided by Biocorp (Goheung-gun, Jeollanam-do, Korea).
Funding
This research was supported by the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Republic of Korea (20220027).
Author information
Authors and Affiliations
Contributions
Investigation and data organization: J-YH; Original draft preparation and visualization: J-YH and MP; Conceptualization and supervision: H-JL. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Jin-Young Han declares that she has no conflict of interest. Miey Park declares that she has no conflict of interest. Hae-Jeung Lee declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, JY., Park, M. & Lee, HJ. Scallop-derived plasmalogen attenuates amyloid beta-induced inflammation and apoptosis in SH-SY5Y cells. Mol. Cell. Toxicol. 20, 421–430 (2024). https://doi.org/10.1007/s13273-023-00399-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00399-2