Abstract
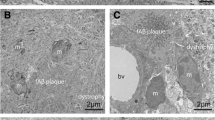
Two distinct species of amyloid β protein (Aβ) with different carboxyl termini, Aβ40 and Aβ42(43), are deposited in plaques in the brains of patients with Alzheimer's disease and Down's syndrome. The relationship between these two forms of Aβ and microglial cells was investigated in 16 subjects with Down's syndrome ranging in age from 31 to 64 years. The amount of Aβ40 in plaques was low in persons under 50 years of age, even though high amounts of Aβ42(43) were present, but this former species increased after this age. Microglia were observed most commonly in plaques containing both Aβ40 and Aβ42(43) but less commonly in those with Aβ42(43) alone. The presence of microglial cells in plaques may be associated with the accumulation of Aβ40 and these cells may have a role in the production or processing of this particular molecular species.
Similar content being viewed by others
References
Bauer J, Konig G, Strauss S, Jonas U, Ganter U, Weidemann A, Monning U, Masters CL, Volk B, Berger M, Beyreuther K (1991) In vitro maturated human macrophages express Alzheimer's βA4-amyloid precursor protein indicating synthesis in microglial cells. FEBS Lett 282: 335–340
Cras P, Kawai M, Siedlak S, Mulvihill P, Gambetti P, Lowery D, Gonzalez-DeWhitt P, Greenberg B, Perry G (1990) Neuronal and microglial involvement in β-amyloid protein deposition in Alzheimer's disease. Am J Pathol 137: 241–246
Dickson D, Lee SC, Mattiace LA, Yen S-H, Brosnan C (1993) Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia 7: 75–83
Fluharty AL, Davis LD, Trammell JL, Stevens RL, Kihara H (1975) Mucopolysaccharides synthesized by cultured glial cells derived from a patient with Sanfilippo A syndrome. J Neurochem 25: 429–435
Grundke-Iqbal I, Fleming J, Tung Y-C, Lassmann H, Iqbal K, Joshi JG (1990) Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol 81: 105–110
Haass C, Hung AY, Selkoe DJ (1991) Processing of β-amyloid precursor protein in microglia and astrocytes favours an internal localization over constitutive secretion. J Neurosci 11: 3783–3793
Hyman BT, Marzloff K, Arriagada PV (1993) The lack of accumulation of senile plaques or amyloid burden in Alzheimer's disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol 52: 594–600
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24: 173–182
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of Aβ42(43)-positive and Aβ40-positive senile plaques with end-specific Aβ monclonal antibodies: evidence that an initially deposited species is Aβ1-42(43). Neuron 13: 45–53
Iwatsubo T, Mann DMA, Odaka A, Suzuki N, Ihara Y (1995) Amyloid β protein (Aβ deposition: Aβ42(43) precedes Aβ40 in Down syndrome. Ann Neurol 37: 294–299
Jarrett JT, Berger EP, Lansbury PT (1993) The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation. Implications for the pathogenesis of Alzheimer's disease. Biochemistry 32: 4693–4697
Maat-Schieman MLC, Rozemuller AJ, Van Duinen SG, Haan J, Eikelenboom P, Roos RAC (1994) Microglia in diffuse plaques in hereditary cerebral haemorrhage with amyloidosis (Dutch). An immunohistochemical study. J Neuropathol Exp Neurol 53: 483–491
Mak K, Yang F, Vinters HV, Frautschy SA, Cole GM (1994) Polyclonals to β amyloid (1–42) identify most plaque and vascular deposits in Alzheimer cortex, but not striatum. Brain Res 667: 138–142
Mann DMA (1994) Alzheimer's disease: progress in pathological and aetiological aspects. Rev Clin Gerontol 4: 43–60
Mann DMA, Younis N, Jones D, Stoddart RW (1992) The time course of pathological events concemed with plaque formation in Down's syndrome with particular reference to the involvement of microglial cells. Neurodegeneration 1: 201–215
Mann DMA, Iwatsubo T, Ihara Y, Odaka A, Suzuki N, Cairns N, Lantos PL, Bogdanovic N, Lannfelt L, Winblad B, Maat-Schieman M (1995) Predominant deposition of Aβ42 in plaques in cases of Alzheimer's disease and hereditary cerebral haemorrhage associated with mutations in the APP gene. Am J Pathol (submitted)
Murphy GM, Forno LS, Higgins L, Scardina JM, Eng LF, Cordell B (1994) Development of a monoclonal antibody specific for the COOH-terminal of β-amyloid 1–42 and its immunohistochemical reactivity in Alzheimer's disease and related disorders. Am J Pathol 144: 1082–1088
Naidu A, Quon D, Cordell B (1995) β-amyloid peptide produced in vitro is degraded by proteinases released by cultured cells. J Biol Chem 270: 1369–1374
Ohgami T, Kitamoto T, Shin R-W, Kaneko Y, Ogomori K, Tateishi J (1991) Increased senile plaques without microglia in Alzheimer's disease. Acta Neuropathol 81: 242–247
Perlmutter LS, Barron E, Chui HC (1990) Morphologic association between microglia and senile plaque amyloid in Alzheimer's disease. Neurosci Lett 119: 32–36
Royston MC, Kodical NS, Mann DMA, Groom K, Landon M, Roberts GW (1994) Quantitative analysis of β-amyloid deposition in Down's syndrome using computerized image analysis. Neurodegeneration 3: 43–51
Rozemuller JM, Bots GTAM, Roos RAC, Eikelenboom P (1992) Acute phase proteins but not activated microglial cells are present in parenchymal β/A4 deposits in the brains of patients with hereditary cerebral haemorrhage with amyloidosis — Dutch-type. Neurosci Lett 140: 137–140
Saido TC, Iwatsubo T, Mann DMA, Shimada H, Ihara Y, Kawashima S (1995) Dominant and differential deposition of distinct β-amyloid peptide species, AbN3(PE) in senile plaques. Neuron 14: 457–466
Savage MJ, Pinsker LR, Emmons T, Kawooya JK, Siman R, Greenberg B (1994) Immunohistochemical evidence that Aβ1--42 is more abundant in amyloid plaques than Aβ1-40 in Alzheimer's disease brain. Neurobiol Ageing 15: S52-S53
Scott SA, Johnson SA, Zarow C, Perlmutter LS (1993) Inability to detect β-amyloid protein precursor mRNA in Alzheimer plaque associated microglia. Exp Neurol 121: 113–118
Selkoe DJ (1994) Normal and abnormal biology of the β amyloid precursor protein. Annu Rev Neurosci 17: 489–517
Snow AD, Mar H, Nochlin D, Kimata K, Kato M, Suzuki S, Hassell J, Wight TN (1988) The presence of heparan sulphate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol 133: 456–463
Snow AD, Mar H, Nochlin D, Sekiguchi RT, Kimata K, Koike Y, Wight TN (1990) Early accumulation of heparan sulphate in neurones and in the β amyloid protein containing lesions of Alzheimer's disease and Down's syndrome. Am J Pathol 137: 1253–1270
Snow AD, Sekiguchi R, Nochlin D, Fraser P, Kimata K, Mizutani A, Arai M, Schreier WA, Morgan DC (1994) An important role of heparan sulphate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar Aβ amyloid in rat brain. Neuron 12: 219–234
Snow AD, Seikiguchi RT, Nochlin D, Kalaria RN, Kimata K (1994) Heparan sulphate proteoglycan in diffuse plaques of hippocampus but not of cerebellum in Alzheimer's disease brain. Am J Pathol 144: 337–347
Threlkeld A, Adler R, Hewitt AT (1989) Proteoglycan biosynthesis by chick embryo retina glial-like cells. Dev Biol 132: 559–568
Wegiel J, Wisniewski HM (1990) The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol 81: 116–124
Wisniewski HM, Wegiel J, Wang KC, Kujawa M, Lach B (1989) Ultrastructural studies of the cells forming amyloid fibres in classical plaques. Can J Neurol Sci 16: 535–542
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mann, D.M.A., Iwatsubo, T., Fukumoto, H. et al. Microglial cells and amyloid β protein (Aβ) deposition: association with Aβ40-plaques. Acta Neuropathol 90, 472–477 (1995). https://doi.org/10.1007/BF00294808
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294808