Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease and the leading cause of dementia worldwide. Different pathologic changes have been introduced to be involved in its progression. Although amyloid-β (Aβ) deposition and tau hyperphosphorylation and aggregation are mainly considered the main characterizations of AD, several other processes are involved. In recent years, several other changes, including alterations in gut microbiota proportion and circadian rhythms, have been noticed due to their role in AD progression. However, the exact mechanism indicating the association between circadian rhythms and gut microbiota abundance has not been investigated yet. This paper aims to review the role of gut microbiota and circadian rhythm in AD pathophysiology and introduces a hypothesis to explain their association.
Similar content being viewed by others
Alzheimer’s disease: an overview
Alzheimer’s disease (AD) is the most common neurodegenerative disorder and the leading cause of dementia worldwide1. AD patients experience a variety of symptoms, including memory loss, cognitive alterations, and behavioral changes2,3. AD-related dementia is linked to neurodegeneration, initially characterized by synaptic and neuronal loss4. These processes are accompanied by microglial cell proliferation5,6, astrogliosis7, and the presence of neurofibrillary tangles composed of hyperphosphorylated tau and dystrophic neurites8,9. More recent studies provide uncovered evidence, suggesting that another component of neurodegenerative AD includes the possibility of interference with the process of adult hippocampus neurogenesis10,11. Additionally, studies in transgenic animal models of AD have shown a marked aberrant process of adult neurogenesis in the hippocampus12,13,14.
Regarding the various neuropathological features of AD, cognitive alterations in AD patients are associated with synaptic injury in the limbic system and neocortex15,16. However, the other main characterization of AD is the progressive deposition of amyloid-β (Aβ) protein which has been linked to mentioned neuropathological changes17,18. Abnormal Aβ accumulation is caused by a disturbance in the imbalance between Aβ production, aggregation, and clearance. Clearance of Aβ is mediated by proteolytic enzymes, such as chaperone molecules such as apoE19, neprilysin20, lysosomal (e.g., autophagy)21, and non-lysosomal routes (e.g., proteasome)22. There are two main forms of AD, including familial and sporadic forms. Although in familial forms of AD, mutations cause an increase in Aβ production or aggregation, in sporadic AD, alterations of the clearance pathways might play a crucial role23. Accumulation of Aβ leads to the generation of Aβ oligomers and fibrils, which are the main components of the Aβ plaques24. However, most evidence suggests that the Aβ oligomers rather than the fibrils mediate the Aβ-induced synapto-toxicity25,26.
Axonal pathology and synaptic loss are probably the key neuropathological changes leading to dementia in AD27,28,29; however, other factors have been identified to be involved. In recent years, new studies have suggested several other mechanisms in other organs involved in the pathophysiology of AD. In this regard, changes in intestinal microbiota have been closely linked to the progression of AD, which will be discussed in the next sections.
Gut microbiota and Alzheimer’s disease
There are billions of colonized microbes in the human gut. Increasing evidence suggests that there is a bidirectional association between the human gut microbiota and the brain, which is known as Microbiota–Gut–Brain Axis (MGBA)30. Gut dysbiosis has been associated with a variety of diseases, especially neurological conditions such as neurodegenerative diseases31,32. In this regard, experimental studies have revealed that gut flora is involved in the regulation of brain functions such as memory and learning33. More importantly, the function and composition of intestinal flora affect the pathophysiology of age-related cognitive impairment and dementia, suggesting its crucial role in the onset and progression of AD34,35,36.
Gut microbiota and brain function
The association between the gut flora and the Central nervous system (CNS) is due to the interaction between the intestine and the brain with each other via the nervous system or chemicals which cross the blood-brain barrier (BBB)37. The gut flora produces chemical substances (i.e., amino acids and monoamines) that reach the neurons of CNS via the vascular and lymphatic system and can affect their activity, with probable influences on behavior38. On the other hand, the gut microbiota is affected by the messages as neurotransmitters sent by the brain39,40. Several communication pathways between the brain and gut have been investigated41. The Vagus nerve plays a central role in the connection between the gut and the autonomic nervous system42. This nerve ends to the brain stem nuclei, which give efferent fibers and receive afferent fibers. In this pathway, stem nuclei may regulate many gut activities and send signals to the other regions of the brain, such as the cortical areas and thalamus43. Additionally, the enteric nervous system can send and receive signals from the CNS via the gut flora44. Also, blood circulation is involved in the exchange between the gut and brain45. Intestinal mucosa and BBB allow the passage of endocrine and immune molecules, the most important of which are hormones and cytokines, which can affect the function of both the gut and brain46. Interestingly, it has been reported that gut microbiota affects the maturation of the endocrine, immune, and nervous system in germ-free mice43. Gut microbiota regulates MGBA through different routes. For instance, these microorganisms can synthesize and release neuromodulators and neurotransmitters, such as biogenic amines (e.g., histamine, serotonin, and dopamine), short-chain fatty acids (SCFAs), and other metabolites produced from amino acids such as GABA or serotonin and tryptophan41. On the other hand, the other possibility for MGBA regulation by gut bacteria is that these microorganisms produce substances that are toxic to the brain, such as ammonia and D-lactic acid47. In addition, during several inflammatory processes, the gut microbiota produces and releases other toxic proteins to the brain, such as host innate immune activators48. Alterations in the mentioned processes, especially immunological processes, can contribute to anxiety, memory impairment, and other cognitive alterations47,49,50. Recent studies reported that these alterations are associated with a variety of neurological conditions, including depression51, drug-resistant epilepsy52, and neurodegenerative diseases, especially AD, Parkinson’s disease, and multiple sclerosis53,54,55.
Alterations of the gut microbiota in AD patients
Analysis of intestinal microbiota in AD patients was first conducted by Cattaneo et al.56. In this study, to evaluate the correlation between gut flora and cognitive impairment, the abundance of bacterial gut microbiota taxa in the feces of healthy controls, patients with cognitive impairment and brain amyloidosis, and patients with cognitive impairment without brain amyloidosis, as well as the levels of inflammatory mediators in their blood were measured. The results of this study revealed that the abundance of Escherichia/Shigella species as pro-inflammatory species were increased in the gut flora of patients with cognitive impairment and brain amyloidosis. In contrast, a decrement in the abundance of anti-inflammatory species Eubacterium rectale was detected. Additionally, their results showed a significant correlation between the abundance of gut flora and levels of pro-inflammatory factors IL-1β, NLRP3, and CXCL2. In another study, the proportion of different intestinal microbiota species in AD patients compared to age and sex matched the healthy controls57. Their results showed that the abundance of bacteria with the ability of butyrate synthesis in the flora of AD patients’ feces was reduced. Additionally, they found that fecal samples from AD patients induce lower expression of p-glycoprotein as a key regulator of intestinal homeostasis in epithelial cells of the intestine. There are several other interesting results indicating the association between gut flora and biochemical parameters in blood samples from AD patients. One of these works shows that adiponectin levels correlate with Faecalibacterium, Acidimicrobiia, Oscillospiraceae, Actinobacteria, Prevotella, and Christensenellaceae R-7. Also, Acidobacteriota is linked to total bilirubin, while Firmicutes, Castellaniella alcaligenes, Acidobacteriales bacterium, Lachnospiraceae, Klebsiella pneumoniae, and Christensenellaceae correlate with the level of CRP in the blood of patients with AD58. Additionally, another study revealed that patients with AD or mild cognitive impairment show an increase in bacterial taxa, including Erysipelotrichales, Erysipelatoclostridiaceae, Patescibacteria, Saccharimonadia, and Saccharimonadales, compared with normal control subjects, which were positively associated with APOE 4, and negatively correlated with memory59.
Gut flora and AD-related pathophysiology
Neuroinflammation plays a crucial role in the progression of AD40. However, recent findings suggest a close association between intestinal microbiota and neuroinflammation60. Bacteroidetes family of Gram-negative bacteria, which constitute a considerable abundance of the gut microbiota, releases a mixture of neurotoxins, mainly pro-inflammatory lipopolysaccharides (LPS), leading to trigger systemic inflammation via the promotion of pro-inflammatory cytokines production61. It has been reported that LPS levels in specimens from AD patients elevated by three times in the hippocampus and two times in the neocortex when compared to healthy controls62,63. It has been elucidated that LPS injection during the development of the brain induces microglial activity leading to elevated levels of the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α64. The association between LPS and AD pathology has been linked to its ability to initiate amyloid fibrillogenesis in co-incubation with Aβ peptide65. Additionally, it has been reported that systemic injection of LPS contributes to Aβ deposition and tau aggregation in APPswe transgenic mice66. Interestingly, Gram-negative Escherichia coli-derived LPS and fragments have been detected in Aβ plaques from AD patients67. On the other hand, gut flora can regulate the levels of microRNAs (miRs), a group of important factors in AD pathophysiology. In this regard, it has been reported that gut bacteria-derived LPS can affect miR levels in AD68. Additionally, it has been demonstrated that the neurotropic herpes simplex virus-1 and Gram-negative bacteria Bacteroides fragilis share a final common pathway of NF-κB and microRNA-146a induction which results in the stimulation of neuroinflammatory pathways69. In another study, Bacteroides fragilis-derived LPS has been shown to cross the BBB into brain-parenchyma and neuronal-cytoplasm through systemic circulation and leads to induce the expression of pro-inflammatory miRs, miR- 146a and miR-155, introduced as a contributor to the onset of AD70.
Regardless of the neuroinflammation, the gut microbiota is involved in the other aspects of AD pathophysiology. Animal studies revealed a correlation between Akkermansia and seven other bacterial genera with the cerebral soluble Aβ42 levels71. Butyricicoccus and Akkermansia, two main regulators of gut barrier integrity72, have been shown to be negatively associated with the levels of pathogenic Aβ42 in the brain. It has been hypothesized that a reduced proportion of these bacteria in gut flora may lead to the LPS influx into the brain in AD, as a leaky gut has been detected in patients with AD73,74. Therefore, an increased proportion of Bacteroides, along with a reduced abundance of Butyricicoccus and Akkermansia, may result in elevated LPS translocation from the intestines into the brain via systemic circulation74.
BMAL1 and Alzheimer’s disease
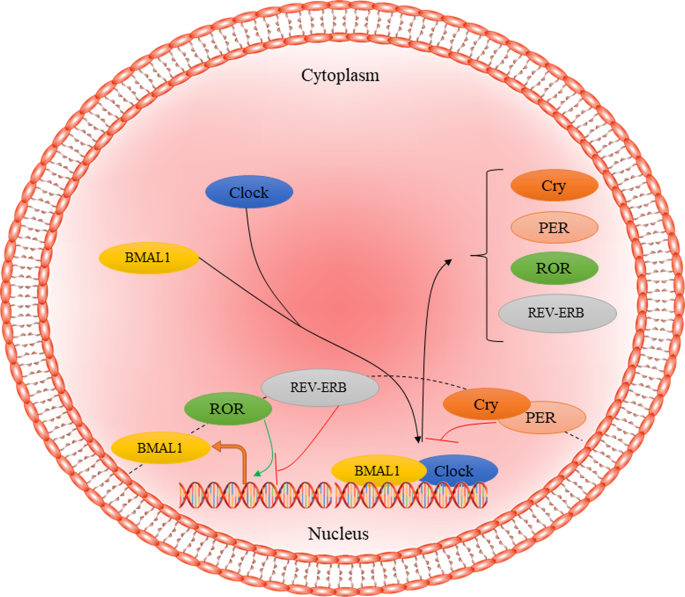
Altered circadian rhythms, irrespective of cause, have been implicated in a multitude of diseases, including metabolic diseases such as obesity75,76, sleep disorders77, psychiatric disorders such as bipolar illness78, and neurodegenerative diseases such as AD79. Brain and muscle Arnt-like protein-1 (BMAL1), encoded by the ARNTL gene, is a core regulator of the circadian clock in humans and is known as the only irreplaceable clock factor regulating rhythmic behaviors80,81. At the molecular inspection, BMAL1 regulates nearly 24 h autonomous circadian oscillations through the transcriptional–translational feedback loop (TTFL). Circadian locomotor output cycles kaput (CLOCK) together with BMAL1 dimerize and bind to the E-box motifs form the positive limb leading to express the cryptochrome (CRY1/2), period (PER1/2/3), retinoid-related orphan receptor-α (RORα), and reverse erythroblastosis virus α (REV-ERBα). Finally, CRY and PER proteins interact with each other, forming cytoplasmic heterodimers, which translocate to the nucleus to inhibit the positive limb expression (Fig. 1)82. On the other hand, RORα and REV-ERBα restrain and facilitate the expression of BMAL1, respectively83. In humans, all fully differentiated cells have this molecular clock based on circadian rhythmicity84. The circadian system is involved in the regulation of different physiological processes, such as the rest-activity cycle, food-intake behavior, and glucose metabolism85. In addition to the circadian system, BMAL1 regulates other aspects of cell survival, such as oxidative response and redox homeostasis, along with nuclear factor-related factor 2 (Nrf2), through regulating the rhythmic expression of Prdx686,87. Further, BMAL1 is involved in regulating inflammatory processes88 and sensitivity to insulin89. It has been reported that ARNTL disruption leads to the progression of aging-related diseases, such as type 2 diabetes mellitus and neurodegenerative diseases90,91.
CLOCK-BMAL1 complex translocates to the nucleus and binds to E-box elements to activate the expression of Cry, PER, RPR, and REV-ERB as the negative regulators of primary complex activity. CRY1/2 cryptochrome, PER period, ROR retinoid-related orphan receptor, REV-ERB reverse erythroblastosis virus α.
One of the most common symptoms of AD is a disrupted circadian rhythm, characterized by awakening and increased sleep during the night and day, respectively92. AD-related loss of the normal circadian rhythm has been widely associated with altered BMAL1 activity93 as aberrant BMAL11 activity and levels have been detected in samples from AD patients. In this regard, it has been demonstrated that patients with AD show a higher prevalence of T carriers in BMAL1 rs.2278749 T/C in comparison with healthy controls in whole blood samples from the antecubital vein94. In addition, different levels of BMAL1 levels from AD patients and healthy controls have been detected in the occipital cortex, frontal cortex, temporal cortex, pineal glands, and parietal cortex95,96. In a molecular inspection, excessive studies have shown a correlation between BMAL1 and different factors involved in the pathophysiology of AD. In this regard, mutual communication between BMAL1 and Aβ deposition has been reported in different studies. The aberrant expression of BMAL1 protein has been proposed to be a result of the Aβ effect in mouse hippocampus97. Additionally, it has been shown that Aβ can accelerate BMAL1 degradation in an animal model of AD98. On the other hand, the BMAL1 loss has been shown to accelerate the Aβ plaques accumulation99. Additionally, BMAL1 is associated with responses of astrocytes and microglia to Aβ deposits. It has been shown that BMAL1 deletion in mice contributes to exacerbated astrocyte activation around Aβ plaques along with altered gene expression100. Also, REV-ERBs inhibition has been shown to increase BMAL1 transcription and induce microglial Aβ phagocytic activity leading to enhance Aβ clearance in the 5XFAD mouse model of AD101.
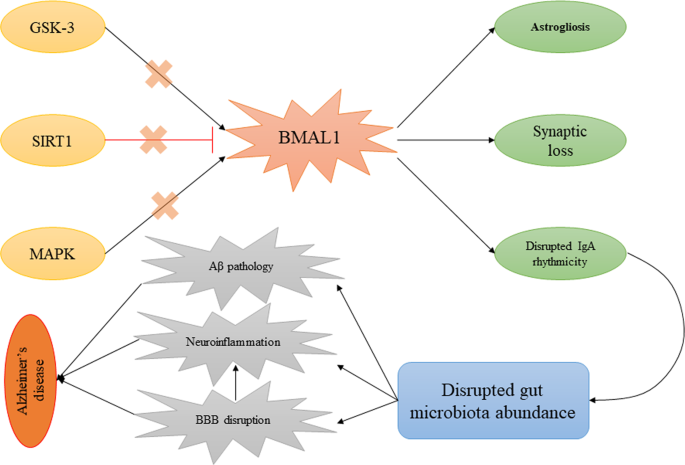
In addition to the mentioned points, altered BMAL1 in astrocytes in different studies revealed several pathological changes that promote AD. In a study, lower cortactin expression, lower Rho-GTP levels, and impaired actin cytoskeleton dynamics were observed in BMAL1−/− astrocytes leading to disrupted synaptic integrity. Also, in this study, BMAL1−/− mice showed a significant decrement of synaptic coverage by astrocytes, which is associated with chronodisruption-induced cognitive deficit102. On the other hand, BMAL1 regulates astrocyte-to-neuron communication via the prevention of GABA accumulation in intracellular space100. In this regard, it was shown that treatment of BMAL1cKO mice with GABA receptor antagonists led to abolished cognitive functions103. Further, deletion of astrocyte-specific BMAL1 led to neuronal death and astrogliosis, a hallmark of AD, leading to enhanced expression of inflammatory genes104. In addition to astrocytes, it has been reported that partial knockdown of BMAL1 in neurons results in impaired structure and dysfunction of synapses and spontaneous neurodegeneration105. There is no idea about the aberrant levels of BMAL1 in AD. In this regard, it can be referred to regulators of BMAL1 with altered activity in AD to explain this disruption. One of the main regulators of BMAL1 is Sirtuin 1 (SIRT1) involved in its regulation through PER2 deacetylation Lower expressions of SIRT1 in samples from AD patients has been detected106,107. Mitogen-activated protein kinase (MAPK), the other altered factor in AD, has been shown to negatively regulate BMAL1 via its phosphorylation at Thr-534108. The other regulator of BMAL1 is glycogen synthase kinase 3 (GSK-3), which plays a crucial role in the pathophysiology of AD via phosphorylation of tau protein109,110. This evidence shows that the disruption of the level of BMAL1 does not happen by itself in AD; as a secondary factor, it can be due to the disruption of its regulators.
A hypothesis on the association between BMAL1, IgA, and intestinal microbiota in Alzheimer’s disease
Many studies reveal that the composition of gut microbiota oscillates rhythmically, mainly in response to several body rhythms such as circadian rhythm111. In a study on mice gut flora, it was reported that a relative abundance of Lactobacillales, Clostridiales, Bacteroidales, Firmicutes, Bacteroidetes, Clostridium spp, Ruminococcaceae spp, Lachnospiraceae spp, Bacteroides, Anaeroplasma, and Lactobacillaceae spp. rhythmically oscillate in a 24-h period, which may be regulated by different factors involved in circadian rhythm111,112. In addition to gut flora abundance, changes in its functionality have been reported in response to altered light-dark rhythmicity. In this case, it has been elucidated that in mice, which exhibit light-dark rhythmicity opposite to humans, gut flora favored energy metabolism, cell growth, and DNA repair during the dark phase of maximum activity111.
Although many studies have shown the association between BMAL1 and AD, most of these studies have dealt with this connection in the CNS. However, the interaction between BMAL1 and gut microbiota has been reported in several studies. In this case, Zhang and colleagues have examined the effect of intermittent photoperiod on gut microbiota abundance in mice113. Their results reveal that intermittent photoperiod (16 [3 h-L/1 h-D]: 8 D) leads to enhance the circadian rhythms of c BMAL1, cBmal2, cCry1, and cCry2 in the hypothalamus and increases the expression of cClock, c BMAL1, and cCry2 in the liver and seven clock genes in the cecal wall. Additionally, they found that these changes eventually resulted in altered composition and metabolic function of the cecal microbiota in a way that the concentrations of SCFAs and the abundance of SCFA-producing genera, such as Odoribacter, significantly increased under the intermittent photoperiod treatment. To explain how circadian rhythm regulators, mainly BMAL1, regulate the abundance and function of gut flora, a recent study by Penny et al.114 showed an interesting result which can be discussed here. In this study, it was shown that the immunoglobulin A (IgA) secretion follows a rhythmic oscillation. Additionally, they found that this rhythmicity in IgA secretion influences gut microbiota abundance. The most interesting point in this study was the regulation of rhythmic IgA secretion and gut microbiota proportion by circadian rhythm, as detected, followed by the deletion of the ARNTL gene. These results may provide new insights into a more detailed description of the role of BMAL1 in AD. It can be said that disturbance of BMAL1 levels in AD followed by alterations in its regulators may lead to the lack of IgA rhythmic secretion and, eventually, altered microbiota abundance. To support these results, altered levels of IgA, as well as elevated numbers of IgA+ cells, have been detected in the cornu ammonis region of AD patients115, which may be due to disturbance of BMAL1 activity in these patients. This issue may explain the probable therapeutic role of intravenous immunoglobulin in AD116, while rhythmic administration of IgA may provide better results in this case. In addition to mentioned points, according to this possible communication, a mechanism can be proposed to explain the BBB disruption in AD. In this regard, it has been shown that disruption of BMAL1 is accompanied by impaired BBB and efflux transport117,118. On the other hand, it has been elucidated that an altered proportion of gut bacteria results in increased BBB leakage119, which may be linked to BMAL1-IgA communication. These processes may explain the therapeutic potential of melatonin on AD progression in different studies, as it has been shown that it modulates SIRT1, MAPK, and GSK-3 and induces BMAL1 activity120. Regardless of the role of these factors, Gao et al.121 found a link between melatonin and intestinal barrier dysfunction in mice. In this study, it was reported that reductions in melatonin levels followed by sleep deprivation contribute to increased pro-inflammatory cytokines, reduced anti-inflammatory cytokines, and colonic mucosal injury. They linked these changes to reduced gut flora diversity, decreased Bacteroides, Akkermansia, and Faecalibacterium, and increased Aeromonas resulting from reduced melatonin levels.
In addition to the role of BMAL1 in the regulation of gut microbiota abundance, the opposite association should also be examined, which can be considered as a regulatory loop between gut microbiota and BMAL1. In this regard, gut microbiota-derived chemicals are shown to be involved in the regulation of circadian rhythms via the regulation of BMAL1 expression. In a study by Leone et al., it was found that treatment of hepatic cells with sodium acetate and sodium butyrate changes the expression of BMAL1 and its regulator, PER2122. In addition, it was observed that treatment with SFCAs, especially butyrate, results in the induction of BMAL1 expression. Figure 2 depicts the hypothesized association between BMAL1 and IgA-gut microbiota in AD.
Aberrant activity of SIRT1, MAPK, and GSK-3 results in altered BMAL1 activity in AD. On the other hand, disruption in BMAL1 activities may contribute to altered IgA secretion and gut microbiota abundance. This process induced Aβ deposition, neuroinflammation, and BBB disruption leading to AD progression. AD Alzheimer’s disease, BMAL1 brain and muscle ARNT-Like 1, GSK-3 glycogen synthase kinase, MAPK mitogen-activated protein kinases, SIRT1 Sirtuin 1.
Future perspective
The regulatory effects of BMAL1 on gut microbiota seem to be mediated by IgA. This connection may explain the results of recent findings on altered IgA levels in AD patients which can be due to aberrant activity of BMAL1. However, the association between BMAL1 with gut microbiota in any way could provide a therapeutic target to slow the progression of AD. Further studies may be required to find other possible mechanisms explaining the connection between BMAL1 and gut flora activity. In addition, altered oscillation secretion of IgA due to aberrant activity of BMAL1 can be compensated therapeutically to block the adverse effects of BMAL1 on gut microbiota abundance. These results can introduce new insights into the therapeutic potential of probiotics in AD patients to normalize the abundance of gut microbiota caused by BMAL1. On the other hand, BMAL1 regulators, such as melatonin, can be investigated to evaluate their effects on AD, possibly via regulation of gut flora abundance. Although different pre-clinical and clinical studies have been conducted to study the protective effects of melatonin on the progression of AD, there are no studies that indicate the association between melatonin, circadian rhythm, and gut flora. In addition, this connection may introduce combination therapy with probiotics and melatonin as a suitable and effective therapeutic option for the management of AD. However, more studies are required to prove these claims.
Conclusion
Based on the mentioned evidence, a hypothesis is provided to explain the association between gut microbiota and circadian rhythm in AD patients. More studies to prove this claim may suggest several other therapeutic interventions to modulate the mentioned communication. For instance, melatonin supplementation and other natural compounds may be considered to modulate this alteration.
References
Ashford, J. W. APOE genotype effects on Alzheimer’s disease onset and epidemiology. J. Mol. Neurosci. 23, 157–165 (2004).
Khachaturian, Z. S. Diagnosis of Alzheimer’s disease. Arch. Neurol. 42, 1097–1105 (1985).
Budson, A. E. & Price, B. H. Memory dysfunction. N. Engl. J. Med. 352, 692–699 (2005).
Terry, R. D., Peck, A., DeTeresa, R., Schechter, R. & Horoupian, D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann. Neurol. 10, 184–192 (1981).
Rogers, J., Luber-Narod, J., Styren, S. D. & Civin, W. H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 9, 339–349 (1988).
Masliah, E. et al. Immunoreactivity of CD45, a protein phosphotyrosine phosphatase, in Alzheimer’s disease. Acta Neuropathol. 83, 12–20 (1991).
Beach, T. G., Walker, R. & McGeer, E. Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia 2, 420–436 (1989).
Trojanowski, J. Q. & LEE, V. M. Y. “Fatal attractions” of proteins: a comprehensive hypothetical mechanism underlying Alzheimer’s disease and other neurodegenerative disorders. Ann. N. Y. Acad. Sci. 924, 62–67 (2000).
Crews, L., Rockenstein, E. & Masliah, E. APP transgenic modeling of Alzheimer’s disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct. Funct. 214, 111–126 (2010).
Boekhoorn, K., Joels, M. & Lucassen, P. J. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 24, 1–14 (2006).
Li, B. et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J. Neuropathol. Exp. Neurol. 67, 78–84 (2008).
Wen, P. H. et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp. Neurol. 188, 224–237 (2004).
Dong, H. et al. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 127, 601–609 (2004).
Chevallier, N. L. et al. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am. J. Pathol. 167, 151–159 (2005).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
DeKosky, S. T., Scheff, S. W. & Styren, S. D. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration 5, 417–421 (1996).
Selkoe, D. J. Amyloid β protein precursor and the pathogenesis of Alzheimer’s disease. Cell 58, 611–612 (1989).
Sisodia, S. S. & Price, D. L. Role of the β‐amyloid protein in Alzheimer’s disease. FASEB J. 9, 366–370 (1995).
Kim, J., Basak, J. M. & Holtzman, D. M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63, 287–303 (2009).
Iwata, N. et al. Metabolic regulation of brain Aβ by neprilysin. Science 292, 1550–1552 (2001).
Bendiske, J. & Bahr, B. A. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis—an approach for slowing Alzheimer disease? J. Neuropathol. Exp. Neurol. 62, 451–463 (2003).
Marambaud, P., Zhao, H. & Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 280, 37377–37382 (2005).
Israel, M. A. et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482, 216–220 (2012).
Selkoe, D. J. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399, A23–A31 (1999).
Walsh, D. M. & Selkoe, D. J. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept. Lett. 11, 213–228 (2004).
Klein, W. L., Krafft, G. A. & Finch, C. E. Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 24, 219–224 (2001).
Raff, M. C., Whitmore, A. V. & Finn, J. T. Axonal self-destruction and neurodegeneration. Science 296, 868–871 (2002).
Masliah, E. Recent advances in the understanding of the role of synaptic proteins in Alzheimer’s disease and other neurodegenerative disorders. J. Alzheimer’s Dis. 3, 121–129 (2001).
Scheff, S. W. & Price, D. A. Alzheimer’s disease-related synapse loss in the cingulate cortex. J. Alzheimer’s Dis. 3, 495–505 (2001).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Scheperjans, F. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358 (2015).
Kong, G. et al. Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 135, 104268 (2020).
Chu, C. et al. The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548 (2019).
Jiang, C., Li, G., Huang, P., Liu, Z. & Zhao, B. The gut microbiota and Alzheimer’s disease. J. Alzheimer’s Dis. 58, 1–15 (2017).
Shen, L. & Ji, H.-F. Associations between gut microbiota and Alzheimer’s disease: current evidences and future therapeutic and diagnostic perspectives. J. Alzheimer’s Dis. 68, 25–31 (2019).
Ticinesi, A., Nouvenne, A., Tana, C., Prati, B. & Meschi, T. Gut microbiota and microbiota-related metabolites as possible biomarkers of cognitive aging. Adv. Exp. Med. Biol. 1178, 129–154 (2019).
Collins, S. M., Surette, M. & Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10, 735–742 (2012).
Wekerle, H. The gut–brain connection: triggering of brain autoimmune disease by commensal gut bacteria. Rheumatology 55, ii68–ii75 (2016).
Briguglio, M. et al. Dietary neurotransmitters: a narrative review on current knowledge. Nutrients 10, 591 (2018).
Calsolaro, V. & Edison, P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 12, 719–732 (2016).
Dinan, T. G. & Cryan, J. F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46, 77–89 (2017).
Bonaz, B., Bazin, T. & Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12, 49 (2018).
Wang, H. X. & Wang, Y. P. Gut microbiota-brain axis. Chin. Med. J. 129, 2373–2380 (2016).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Logsdon, A. F., Erickson, M. A., Rhea, E. M., Salameh, T. S. & Banks, W. A. Gut reactions: how the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. 243, 159–165 (2018).
Zac-Varghese, S., Tan, T. & Bloom, S. R. Hormonal interactions between gut and brain. Discov. Med. 10, 543–552 (2010).
Galland, L. The gut microbiome and the brain. J. Med. Food 17, 1261–1272 (2014).
Alam, R., Abdolmaleky, H. M. & Zhou, J. R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med. Genet. B Neuropsychiatr. Genet. 174, 651–660 (2017).
Johnson, K. V. & Foster, K. R. Why does the microbiome affect behaviour? Nat. Rev. Microbiol. 16, 647–655 (2018).
Gareau, M. G. et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317 (2011).
Lach, G., Schellekens, H., Dinan, T. G. & Cryan, J. F. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15, 36–59 (2018).
Braakman, H. M. H. & van Ingen, J. Can epilepsy be treated by antibiotics? J. Neurol. 265, 1934–1936 (2018).
Quigley, E. M. M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 17, 94 (2017).
Kirby, T. O. & Ochoa-Repáraz, J. The gut microbiome in multiple sclerosis: a potential therapeutic avenue. Med. Sci. 6, 69 (2018).
Barichella, M. et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 34, 396–405 (2019).
Cattaneo, A. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68 (2017).
Haran, J. P. et al. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio 10, e00632–00619 (2019).
Kaiyrlykyzy, A. et al. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 12, 15115 (2022).
Zhu, Z. et al. Altered gut microbiota and its clinical relevance in mild cognitive impairment and Alzheimer’s disease: Shanghai Aging Study and Shanghai Memory Study. Nutrients 14, 3959 (2022).
Sochocka, M. et al. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol. Neurobiol. 56, 1841–1851 (2019).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Zhao, Y., Jaber, V. & Lukiw, W. J. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 7, 318 (2017).
Zhao, Y., Cong, L., Jaber, V. & Lukiw, W. J. Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front. Immunol. 8, 1064 (2017).
Cai, Z., Pan, Z. L., Pang, Y., Evans, O. B. & Rhodes, P. G. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr. Res. 47, 64–72 (2000).
Asti, A. & Gioglio, L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J. Alzheimers. Dis. 39, 169–179 (2014).
Sheng, J. G. et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol. Dis. 14, 133–145 (2003).
Zhan, X. et al. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87, 2324–2332 (2016).
Hewel, C. et al. Common miRNA patterns of Alzheimer’s disease and Parkinson’s disease and their putative impact on commensal gut microbiota. Front. Neurosci. 13, 113 (2019).
Zhao, Y. & Lukiw, W. J. Microbiome-mediated upregulation of microRNA-146a in sporadic Alzheimer’s disease. Front. Neurol. 9, 145 (2018).
Alexandrov, P., Zhai, Y., Li, W. & Lukiw, W. Lipopolysaccharide-stimulated, NF-kB-, miRNA-146a- and miRNA-155-mediated molecular-genetic communication between the human gastrointestinal tract microbiome and the brain. Folia Neuropathol. 57, 211–219 (2019).
Harach, T. et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7, 41802 (2017).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Leblhuber, F., Geisler, S., Steiner, K., Fuchs, D. & Schütz, B. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J. Neural Transm. 122, 1319–1322 (2015).
Li, Z., Zhu, H., Zhang, L. & Qin, C. The intestinal microbiome and Alzheimer’s disease: a review. Animal Model Exp. Med. 1, 180–188 (2018).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 (2005).
Froy, O. & Garaulet, M. The circadian clock in white and brown adipose tissue: mechanistic, endocrine, and clinical aspects. Endocr. Rev. 39, 261–273 (2018).
Ma, W. et al. Chronic paradoxical sleep deprivation-induced depressionlike behavior, energy metabolism and microbial changes in rats. Life Sci. 225, 88–97 (2019).
Takaesu, Y. Circadian rhythm in bipolar disorder: a review of the literature. Psychiatry Clin. Neurosci. 72, 673–682 (2018).
Canevelli, M. et al. Sundowning in dementia: clinical relevance, pathophysiological determinants, and therapeutic approaches. Front. Med. 3, 73 (2016).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Welz, P. S. et al. BMAL1-driven tissue clocks respond independently to light to maintain homeostasis. Cell 177, 1436–1447.e1412 (2019).
Richards, J. & Gumz, M. L. Mechanism of the circadian clock in physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R1053–R1064 (2013).
Peng, X. et al. A growing link between circadian rhythms, type 2 diabetes mellitus and Alzheimer’s disease. Int. J. Mol. Sci. 23, 504 (2022).
Reinke, H. & Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 20, 227–241 (2019).
Stenvers, D. J., Scheer, F., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Chhunchha, B., Kubo, E. & Singh, D. P. Clock protein Bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6. Cells 9, 1861 (2020).
Xie, M. et al. BMAL1-downregulation aggravates Porphyromonas gingivalis-induced atherosclerosis by encouraging oxidative stress. Circ. Res. 126, e15–e29 (2020).
Liu, W. W. et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J. 34, 6570–6581 (2020).
Shi, S. Q., Ansari, T. S., McGuinness, O. P., Wasserman, D. H. & Johnson, C. H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 23, 372–381 (2013).
Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010).
Musiek, E. S. & Holtzman, D. M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008 (2016).
Mattis, J. & Sehgal, A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol. Metab. 27, 192–203 (2016).
Fan, R. et al. Importance of Bmal1 in Alzheimer’s disease and associated aging-related diseases: mechanisms and interventions. Aging Cell 21, e13704 (2022).
Chen, Q., Peng, X. D., Huang, C. Q., Hu, X. Y. & Zhang, X. M. Association between ARNTL (BMAL1) rs2278749 polymorphism T >C and susceptibility to Alzheimer disease in a Chinese population. Genet. Mol. Res. 14, 18515–18522 (2015).
Yoo, I. D. Elevated CLOCK and BMAL1 contribute to the impairment of aerobic glycolysis from astrocytes in Alzheimer’s disease. Int. J. Mol. Sci. 21, 7862 (2020).
Cermakian, N., Lamont, E. W., Boudreau, P. & Boivin, D. B. Circadian clock gene expression in brain regions of Alzheimer’s disease patients and control subjects. J. Biol. Rhythms 26, 160–170 (2011).
Wang, Y. et al. Disruption of the circadian clock alters antioxidative defense via the SIRT1-BMAL1 pathway in 6-OHDA-induced models of Parkinson’s disease. Oxid. Med. Cell. Longev. 2018, 4854732 (2018).
Song, H. et al. Aβ-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol. Neurodegener. 10, 13 (2015).
Kress, G. J. et al. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 215, 1059–1068 (2018).
McKee, C. A. et al. Astrocytes deficient in circadian clock gene Bmal1 show enhanced activation responses to amyloid-beta pathology without changing plaque burden. Sci. Rep. 12, 1796 (2022).
Lee, J. et al. Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer’s disease. Aging Cell 19, e13078 (2020).
Ali, A. A. H. et al. Bmal1-deficiency affects glial synaptic coverage of the hippocampal mossy fiber synapse and the actin cytoskeleton in astrocytes. Glia 68, 947–962 (2020).
Barca-Mayo, O. et al. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat. Commun. 8, 14336 (2017).
Lananna, B. V. et al. Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep. 25, 1–9.e5 (2018).
Musiek, E. S. et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 123, 5389–5400 (2013).
Hadar, A. et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s disease. Sci. Rep. 8, 8465 (2018).
Asher, G. et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 (2008).
Sanada, K., Okano, T. & Fukada, Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J. Biol. Chem. 277, 267–271 (2002).
Khezri, M. R., Yousefi, K., Esmaeili, A. & Ghasemnejad-Berenji, M. The role of ERK1/2 pathway in the pathophysiology of Alzheimer’s disease: an overview and update on new developments. Cell. Mol. Neurobiol. 43, 177–191 (2023).
Sahar, S., Zocchi, L., Kinoshita, C., Borrelli, E. & Sassone-Corsi, P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS ONE 5, e8561 (2010).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Liang, X., Bushman, F. D. & FitzGerald, G. A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl Acad. Sci. USA 112, 10479–10484 (2015).
Zhang, Y. et al. Reducing light exposure enhances the circadian rhythm of the biological clock through interactions with the gut microbiota. Sci. Total Environ. 858, 160041 (2023).
Penny, H. A. et al. Rhythmicity of intestinal IgA responses confers oscillatory commensal microbiota mutualism. Sci. Immunol. 7, eabk2541 (2022).
Pocevičiūtė, D. et al. Increased plasma and brain immunoglobulin A in Alzheimer’s disease is lost in apolipoprotein E ε4 carriers. Alzheimer’s Res. Ther. 14, 117 (2022).
Dodel, R. et al. Intravenous immunoglobulins as a treatment for Alzheimer’s disease. Drugs 70, 513–528 (2010).
Pulido, R. S. et al. Neuronal activity regulates blood-brain barrier efflux transport through endothelial circadian genes. Neuron 108, 937–952. e937 (2020).
Medina-Flores, F. et al. Sleep loss disrupts pericyte-brain endothelial cell interactions impairing blood-brain barrier function. Brain Behav. Immun. 89, 118–132 (2020).
Liu, S., Gao, J., Liu, K. & Zhang, H. L. Microbiota-gut-brain axis and Alzheimer’s disease: implications of the blood-brain barrier as an intervention target. Mech. Ageing Dev. 199, 111560 (2021).
Vincent, B. Protective roles of melatonin against the amyloid-dependent development of Alzheimer’s disease: a critical review. Pharmacol. Res. 134, 223–237 (2018).
Gao, T. et al. Role of melatonin in sleep deprivation‐induced intestinal barrier dysfunction in mice. J. Pineal Res. 67, e12574 (2019).
Leone, V. et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, 681–689 (2015).
Author information
Authors and Affiliations
Contributions
M.R.K.: writing manuscript and visualization; M.G.-B.: supervisions, final review, and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khezri, M.R., Ghasemnejad-Berenji, M. Gut microbiota and circadian rhythm in Alzheimer’s disease pathophysiology: a review and hypothesis on their association. npj Aging 9, 9 (2023). https://doi.org/10.1038/s41514-023-00104-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-023-00104-6